Professor Pearse Keane explains how using artificial intelligence (AI) to investigate the eye's retinal tissue can help diagnose dementia.

What is your primary field of research?
I’m a consultant ophthalmologist at Moorfields Eye Hospital and a Professor of Artificial Medical Intelligence at UCL Institute of Ophthalmology.
Although I’m not a computer scientist or engineer, I lead a multidisciplinary research group which aims to develop and apply artificial intelligence in healthcare, both in the UK and globally, using ophthalmology as an exemplar. We cover the full spectrum of research in clinical AI, going “from idea to algorithm” and “from code to clinic”.
What can detailed images of the inside of the eye tell us about dementia at large?
For more than 100 years, doctors have known that the eye can be used as a window to the rest of the body. This makes sense because the retinal blood vessels are the only microvasculature that can be directly visualised in living human subjects.
And similarly, the retinal tissue is the only part of the central nervous system that can be directly seen without removing part of the skull. It’s an approachable part of the brain! From a number of studies, we have evidence showing that signs of degeneration in the retinal tissue may be seen in patients with dementia.
How have developments in artificial intelligence (AI) and machine learning affected your field of research and how did you become involved in the use of these tools?
Our ability to use the eye as a window to systemic health has been supercharged in recent years by the combination of: 1) big data, 2) the latest advances in artificial intelligence, and 3) advances in retinal imaging such as optical coherence tomography.
This has led to a new term being coined, “oculomics”, to describe an emerging field of study. AI can allow us to find new information hidden within retinal images that is not currently visible to human experts.
Have there been any big advancements in AI and 'oculomics' in the past year?
Over the past year, the research team I lead across the UCL Institute of Ophthalmology and Moorfields has made some exciting advances in Oculomics. Perhaps the most important has been the development of RETFound, one of the first AI foundation models in healthcare, and the first in ophthalmology.
RETFound was trained with a curated dataset of 1.6 million images from Moorfields Eye Hospital, using AI tools and infrastructure provided by INSIGHT, the NHS-led health data research hub for eye health based at Moorfields.
We have made RETFound open-source, freely available for any institution worldwide to us, to act as a cornerstone for global efforts to detect and treat blindness using AI.
RETFound could help improve diagnosis of some of the most debilitating eye diseases, including diabetic retinopathy and glaucoma, and predict systemic diseases such as Parkinson’s, stroke and heart failure. We are also working to build on RETFound with even larger and richer training datasets.
You co-lead a research study with Dr Siegfried Wagner called AlzEye, which aims to use eye scans to detect neurodegenerative diseases, what progress has been made in the field of dementia detection?
The AlzEye study is a research study involving UCL and Moorfields Eye Hospital. In it, we have linked ophthalmic imaging data from Moorfields with a national NHS database called Hospital Episode Statistics (HES).
This means that we can identify patients that have had retinal scans done at Moorfields and who have subsequently developed a range of cardiovascular and neurodegenerative diseases.
We used the AlzEye dataset to produce a landmark study, using AI to detect Parkinson’s Disease in retinal biomarkers up to seven years before diagnosis. Led by Siegfried Wagner, the study was published in Neurology, and covered by over 400 international news sources, including the BBC.
Now we are working to build on AlzEye, with the goal of updating the dataset every year, and establish lifetime linkages with HES to create the possibility for very robust longitudinal studies exploring neurological and other systemic conditions, such as dementia.
What is the potential of this work for public health?
If we can find strong signs of systemic disease from eye examinations, it could have huge public health potential.
For example, we know that only a small percentage of people in their 40s and 50s attend their GPs for the NHS Health Check. By contrast, however, the majority of these people will attend their local optometrists for regular eye tests. If we can use such tests to pick up the earliest signs of systemic diseases, it would be transformative!
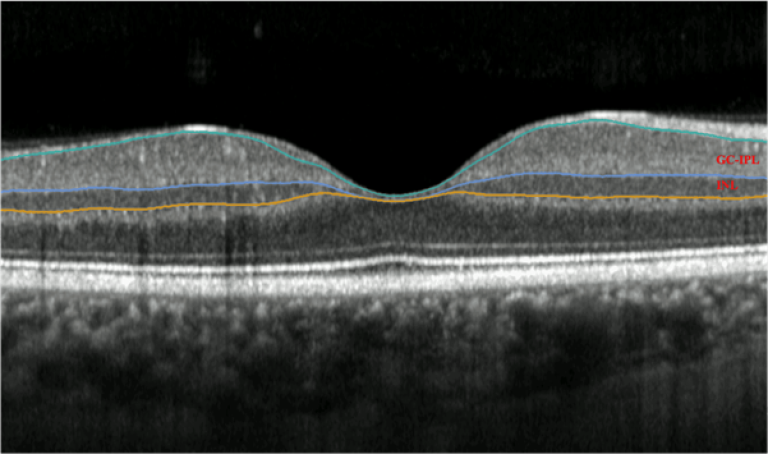
Image: Optical Coherence Tomography (OCT) scan of the retina at the back of the eye, with the layers of the retina highlighted in colour.
 Close
Close

