Euro Ewing 2012 - International Randomised Controlled Trial for the Treatment of Newly Diagnosed Ewing's Sarcoma Family of Tumours
*** Important update: 8 May 2019***
The R1 stage of the Euro Ewing 2012 trial has now closed to recruitment.
The aim of the Euro Ewing 2012 trial is to compare different chemotherapy regimens to determine which is more effective and/or has fewer side effects. 600 patients will be recruited from across Europe from 2013.
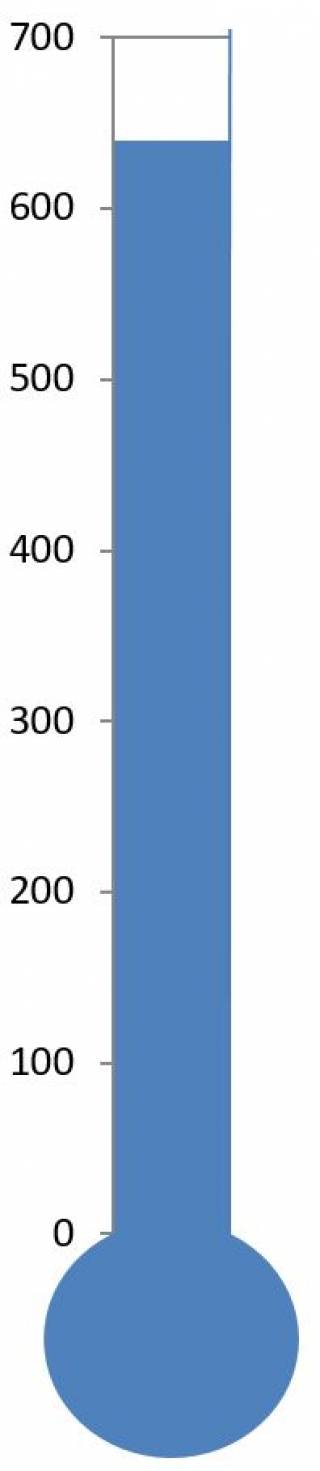
The Euro Ewing 2012 trial has recruited beyond the target of 600 patients and has now closed to recruitment for the R1 stage.
- 640 patients have been recruited so far
- Over 90% of patients have consented to the use of their blood and tissue samples for biological research studies
- As of December 2018, we have collected over 3800 of the following biological samples: blood at diagnosis, frozen tumour tissue, preserved* tumour tissue
- Laboratory research on these patient samples is underway and research published
(*preserved by embedding in paraffin)
Before any results can be released, we have to wait until we have recruited all the 600 patients and followed them for a period of time to see how they get on with treatment. The data is reviewed regularly by independent bodies to check for any safety concerns. Each person who takes part in the trial provides invaluable data and provides an important contribution Ewing sarcoma research.
We are grateful to those patients who have agreed to join this trial, and to their families, and treating clinical teams across Europe.
This page will be updated twice a year.
 Close
Close

