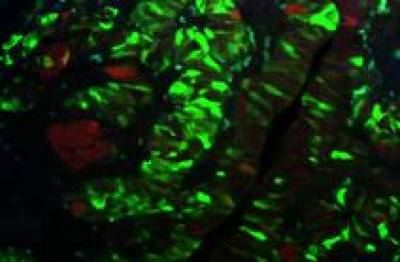
My research program aims to understand the role of cellular senescence in paediatric brain and pituitary tumours and reveal novel therapies against these neoplasias. Paediatric cancers are embryonic defects and as such, we believe that they need to be studied within the context of a developing embryo. My lab combines developmental, molecular and cellular approaches to study high- and low-grade gliomas in children as well as craniopharyngioma, the most common childhood pituitary tumour.
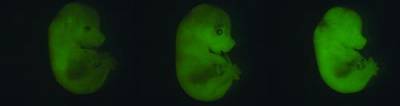
We have developed mouse models of paediatric tumours and performed detailed ‘omics ‘ studies in human and mouse tumours. These approaches have provided important insights into normal brain and pituitary development in the last years (e.g. Haston et al., Development 2017; Carreno et al., Development 2017).
In addition, we have contributed significantly to the understanding of the aetiology and pathogenesis of human congenital hypopituitarism and paediatric craniopharyngioma (Gaston et al., PNAS 2011; Andoniadou et al., Acta Neuropathol. 2012; Andoniadou et al., Cell Stem Cell 2013).
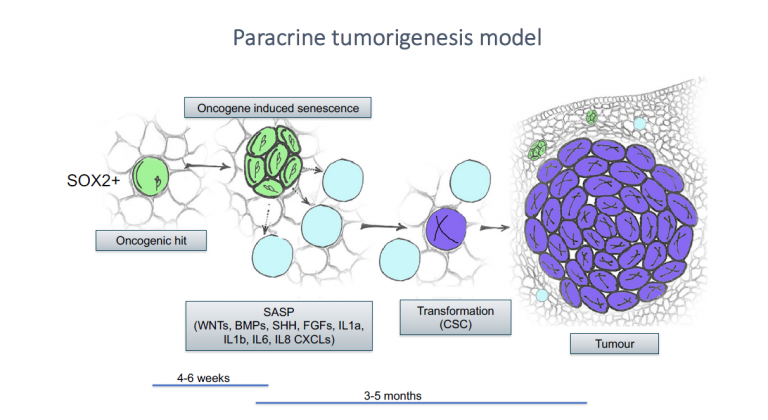
We have revealed that cellular senescence through its secretome drives cell transformation and tumour initiation in the context of craniopharyngioma and that the senescent cells are sensitive to senolytics (Gonzalez-Meljem et al., Nat. Commun. 2017; Guerrero et al., Nat. Metabolism 2019).
We have performed transcriptomic analysis in human samples to identify targetable pathways, and propelled these findings into a clinical trial (Apps et al., Acta Neuropathol. 2018).
More recently, we have expanded our research to other paediatric brain tumours. For instance, using an in vitro model, we have shown that pilocytic astrocytoma, the most frequent brain tumour in children, is sensitive to senolytics (i.e. compounds that selectively kill senescent cells) (Buhl et al., Clinical Cancer Research 2019). Likewise, we are studying models of high-grade glioma to reveal novel vulnerabilities and assess the potential use of senolytics in combination with standard therapies.
- Selected Publications
Gonzalez-Meljem JM et al. Adamantinomatous craniopharyngioma as a model to understand paracrine and senescence-induced tumourigenesis. Cell Mol Life Sci. 2021 PMID: 34019103 Review.
Carreno G et al. Cell senescence in neuropathology: A focus on neurodegeneration and tumours. Neuropathol Appl Neurobiol. 2021. PMID: 33378554 Review.
Apps JR et al. CTNNB1 mutations are clonal in adamantinomatous craniopharyngioma. Neuropathol Appl Neurobiol. 2020. PMID: 32125720.
Guerrero A, et al. Galactose-modified duocarmycin prodrugs as senolytics. Aging Cell. 2020 PMID: 32175667.
Müller HL, et al. Craniopharyngioma. Nat Rev Dis Primers. 2019. PMID: 31699993 Review.
Carreno G et al. SHH pathway inhibition is protumourigenic in adamantinomatous craniopharyngioma. Endocr Relat Cancer. 2019. PMID: 30645190
Guerrero A et al. Cardiac glycosides are broad-spectrum senolytics. Nat Metab. 2019. PMID: 31799499
Apps JR et al. Tumour compartment transcriptomics demonstrates the activation of inflammatory and odontogenic programmes in human adamantinomatous craniopharyngioma and identifies the MAPK/ERK pathway as a novel therapeutic target. Acta Neuropathol. 2018. PMID: 29541918
Gonzalez-Meljem, JM et al. Stem cell senescence drives age-attenuated induction of pituitary tumours in mouse models of paediatric craniopharyngioma. Nature Commun. 2017. PMID: 29180744
Haston, S et al. MAPK pathway activation in the embryonic pituitary results in stem cell compartment expansion, differentiation defects and provides insights into the pathogenesis of papillary craniopharyngioma. Development 2017. PMID: 28506993
Andoniadou, CL et al. Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. 2013
 Close
Close