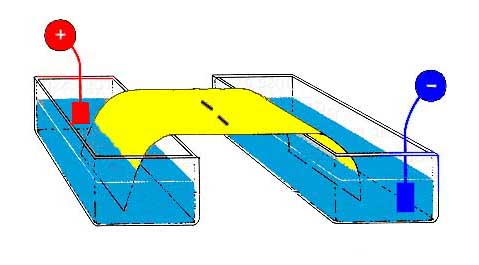
 Electrophoresis
is a technique for separating molecules on the basis of their charge and size.
At an appropriate pH, the distance they move in an electric field depends on
both their overall charge and also their size.
Electrophoresis
is a technique for separating molecules on the basis of their charge and size.
At an appropriate pH, the distance they move in an electric field depends on
both their overall charge and also their size. Electrophoresis
is a technique for separating molecules on the basis of their charge and size.
At an appropriate pH, the distance they move in an electric field depends on
both their overall charge and also their size.
Electrophoresis
is a technique for separating molecules on the basis of their charge and size.
At an appropriate pH, the distance they move in an electric field depends on
both their overall charge and also their size.
The support medium for electrophoresis may be a strip of paper or cellulose acetate (as, for example when small peptides are to be separated), or a gel, such as polyacrylamide (as is commonly used for electrophoresis of proteins and nucleic acids).
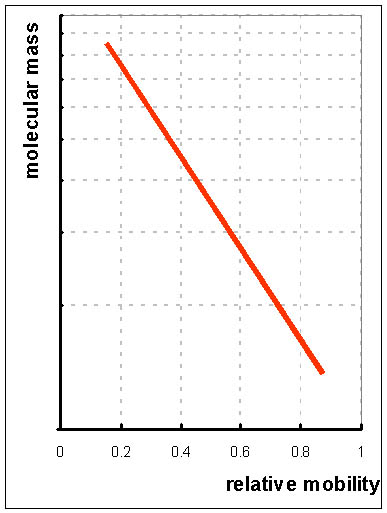
If the protein is denatured with mercaptoethanol (to reduce disulphide bridges to -SH groups) and the anionic detergent sodium dodecyl sulphate (SDS), it becomes coated with a layer of the detergent, and therefore all proteins will have a similar charge. The distance they move on electrophoresis now depends on their size.
Therefore, electrophoresis on polyacrylamide gel after denaturation with SDS (known as SDS-PAGE) together with standards of known molecular mass permits determination of the molecular mass of a protein. Since the protein has been denatured, it has lost all secondary, tertiary and quaternary structure, and if the native protein consists of more than one subunit, these will be separated, and will migrate in the electric field according to their size.

 A polyacrylamide
gel is prepared by co-polymerising acrylamide and methylene bis-acrylamide;
the reaction is initiated by a source of radicals (typically ammonium persulphate,
but sometimes riboflavin and uv irradiation) and the catalyst tetramethylene
ethylene diamine (TEMED).
A polyacrylamide
gel is prepared by co-polymerising acrylamide and methylene bis-acrylamide;
the reaction is initiated by a source of radicals (typically ammonium persulphate,
but sometimes riboflavin and uv irradiation) and the catalyst tetramethylene
ethylene diamine (TEMED).
Methylene bis-acrylamide forms cross-links between the chains of polyacrylamide.
The percentage of methylene bis-acrylamide in the mixture, and hence the extent of cross-linking, determines the size of the pores in the gel, and hence the extent to which proteins of different size are retarded in the electric field.
Smaller proteins move faster.