Measurement of oxygen consumption - the Clark oxygen electrode
 The
utilisation of oxygen during the reaction is measured using an oxygen electrode,
which determines the percentage saturation of oxygen in the reaction mixture.
The instrument is calibrated against a buffer saturated with oxygen to set 100%
saturation and a buffer totally depleted of oxygen (by reaction with a strong
reducing agent such as sodium dithionite) to set 0% saturation.
The
utilisation of oxygen during the reaction is measured using an oxygen electrode,
which determines the percentage saturation of oxygen in the reaction mixture.
The instrument is calibrated against a buffer saturated with oxygen to set 100%
saturation and a buffer totally depleted of oxygen (by reaction with a strong
reducing agent such as sodium dithionite) to set 0% saturation.
The reaction chamber is separated from the electrodes by a teflon membrane,
which permits oxygen to diffuse from the reaction buffer into the potassium
chloride solution that bathes the electrodes: a platinum cathode and a silver
anode. A voltage is applied between the electrodes and the resulting current
(approx. 1 µA) is proportional to the concentration of oxygen.
At the cathode oxygen is reduced to water: 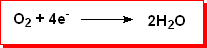
At the anode metallic silver is oxidised to silver chloride: 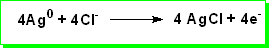
 After
allowing the buffer and substrate to equilibrate, the mitochondrial preparation
is added through the injection port, and the consumption of oxygen is measured
for a short time, then the ADP and any inhibitors, etc, are added, and oxygen
consumption measured until there is no further reaction. The results from a
typical experiment are shown here:
After
allowing the buffer and substrate to equilibrate, the mitochondrial preparation
is added through the injection port, and the consumption of oxygen is measured
for a short time, then the ADP and any inhibitors, etc, are added, and oxygen
consumption measured until there is no further reaction. The results from a
typical experiment are shown here:
In the electrode you will be using in these studies,
100% saturation with oxygen = 1327 nmol O.
 The
utilisation of oxygen during the reaction is measured using an oxygen electrode,
which determines the percentage saturation of oxygen in the reaction mixture.
The instrument is calibrated against a buffer saturated with oxygen to set 100%
saturation and a buffer totally depleted of oxygen (by reaction with a strong
reducing agent such as sodium dithionite) to set 0% saturation.
The
utilisation of oxygen during the reaction is measured using an oxygen electrode,
which determines the percentage saturation of oxygen in the reaction mixture.
The instrument is calibrated against a buffer saturated with oxygen to set 100%
saturation and a buffer totally depleted of oxygen (by reaction with a strong
reducing agent such as sodium dithionite) to set 0% saturation. After
allowing the buffer and substrate to equilibrate, the mitochondrial preparation
is added through the injection port, and the consumption of oxygen is measured
for a short time, then the ADP and any inhibitors, etc, are added, and oxygen
consumption measured until there is no further reaction. The results from a
typical experiment are shown here:
After
allowing the buffer and substrate to equilibrate, the mitochondrial preparation
is added through the injection port, and the consumption of oxygen is measured
for a short time, then the ADP and any inhibitors, etc, are added, and oxygen
consumption measured until there is no further reaction. The results from a
typical experiment are shown here: