The enzymic method that will be used to determine the amino and carboxyl terminal amino acids in this simulation can only determine the terminal and penultimate amino acids with accuracy, so we need to break the peptide into smaller fragments that can be analysed.
Two enzymes can be used to cleave the peptide, and you should use both, to give you two sets of peptide fragments:
At any given pH, the side-chains of some amino acids will be charged, and others not.

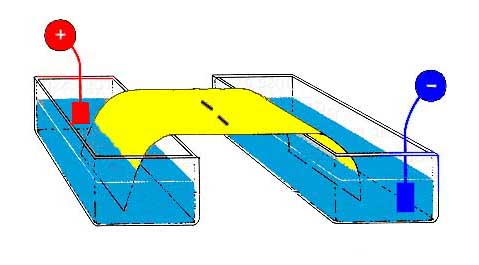 This
means that the overall charge on a peptide varies with pH, and compounds can
be separated on the basis of their charge by the rate at which they migrate
in an electric field. This is the technique of electrophoresis.
This
means that the overall charge on a peptide varies with pH, and compounds can
be separated on the basis of their charge by the rate at which they migrate
in an electric field. This is the technique of electrophoresis.
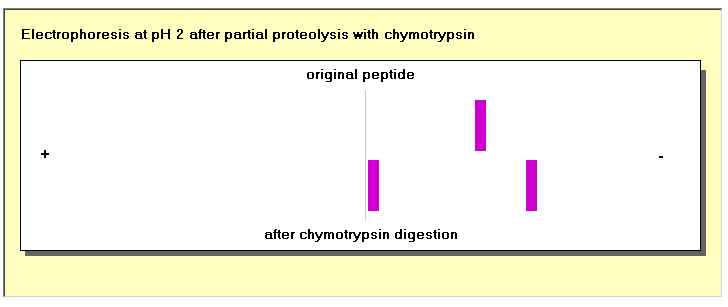
In these studies the peptides to be separated are applied to the origin at the centre of a cellulose acetate strip that is then moistened with buffer and placed between electrodes with a 150 volt potential difference for 1 hour. After this the strip is dried and reacted with ninhydrin to stain the bands that contain the peptides. Selecting the appropriate pH to separate unknown peptides is a matter of trial and error, and in this exercise you may repeat the electrophoresis as often as you wish, with different buffers, until you achieve a separation. You could then separate large quantities of the peptides by electrophoresis, and use them for further studies.
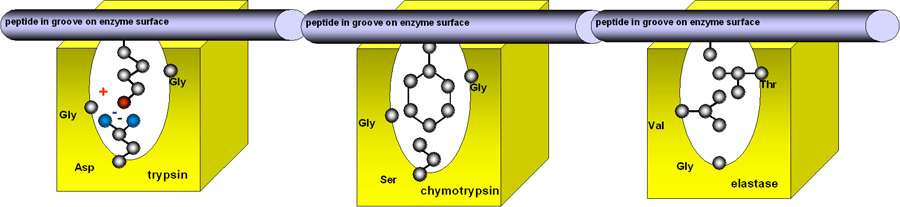
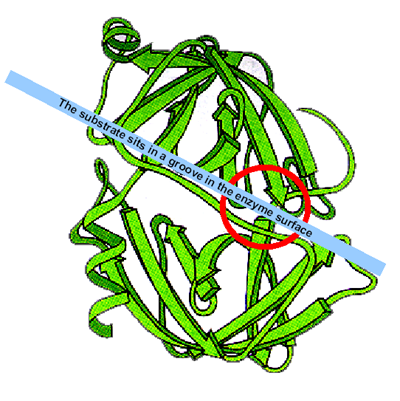 Trypsin,
chymotrypsin and elastase are closely related enzymes. The peptide substrate
sits in a groove in the enzyme surface, with the peptide bond that is to be
hydrolysed over the catalytic site (shown here as a red circle).
Trypsin,
chymotrypsin and elastase are closely related enzymes. The peptide substrate
sits in a groove in the enzyme surface, with the peptide bond that is to be
hydrolysed over the catalytic site (shown here as a red circle).
The amino acid providing the carboxyl group of the bond to be cleaved sits in a pocket below the catalytic site.
In trypsin, which catalyses the hydrolysis of the esters of basic amino acids, the base of this pocket contains an acidic amino acid, aspartate, so providing a negative charge to attract in a basic amino acid side chain.
In chymotrypsin, which catalyses the hydrolysis of the esters of aromatic amino aids, the pocket is lined with small neutral amino acids, leaving room for a bulky aromatic side chain to enter.
In elastase, which catalyses hydrolysis of the esters of small neutral amino acids, the pocket contains two large neutral amino acids, so only a small side chain can fit in.