Aminopeptidase hydrolyses the peptide bond of the amino acid at the amino terminal of a protein or peptide, releasing a free amino acid.
Carboxypeptidase hydrolyses the peptide bond of the amino acid at the carboxyl terminal of a protein or peptide, again releasing a free amino acid.
As the enzymes act, so they expose a new terminal that can act as a substrate.This means that a relatively brief incubation of a peptide one of the enzymes will result in release of mainly the terminal amino acid, with a small amount of the penultimate amino acid. A somewhat longer incubation results in the release of a large amount of the penultimate amino acid. Unfortunately, if incubations continue too long the results become very unclear, so this method can only be used for the terminal and penultimate amino acids.
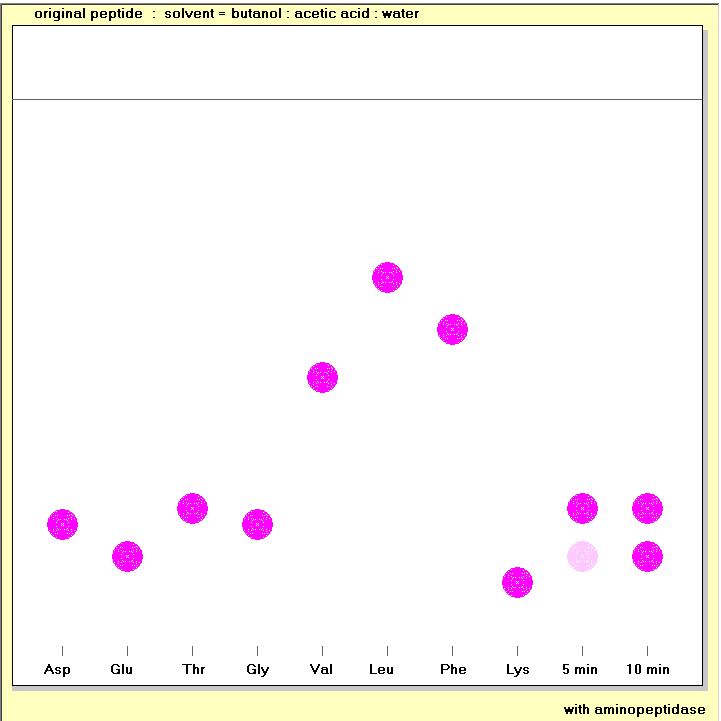
The amino acids that are released by the action of aminopeptidase and carboxypeptidase action can be separated and identified by thin layer chromatography. Chromatography depends on the partition of solutes between a stationary phase (in this case a thin layer of silica gel) and a mobile phase (in this case a liquid). Solutes that are more tightly adsorbed onto the stationary phase travel more slowly; the distance they travel, relative to the solvent front (the Rf value) depends on the chemistry of the solutes, the nature of the stationary phase and the composition of the mobile phase.
In these studies you will be using silica gel thin layer plates. The samples are applied to origins about 1 cm above the base of the plate, and the chromatogram is developed by placing the thin layer plate in a tank containing solvent at the bottom ( in equilibrium with solvent vapour above), and allowing the solvent to rise up the plate by capillary action. With a 10 cm thin layer plate the solvent front rises to near the top of the plate within about an hour. After drying the plate it is sprayed with ninhydrin solution; the ninhydrin reacts with amino acids to yield a purple derivative.

In these studies you will determine the amino acids released from the original peptide, and the fragments produced by trypsin and chymotrypsin action, by incubation with aminopeptidase and carboxypeptidase, each acting for both 5 minutes (when mainly the terminal amino acid is released) and 10 minutes (when the penultimate amino acid is also released).
You will need to decide which standard amino acids to spot onto the chromatography plate in order to identify the terminal and penultimate amino acids - your results from hplc of the total hydrolysate should tell you which standards to use.
Some pairs of amino acids do not separate well in some solvents. There are 5 different solvents available for the chromatography exercise; if the first one you select does not give clear results, try another, until you are confident of your identification of the terminal and penultimate amino acids.
You should now be able to work out the amino acid sequence of the peptide fragments produced by trypsin and chymotrypsin action, and hence the amino acid sequence of the complete peptide.
