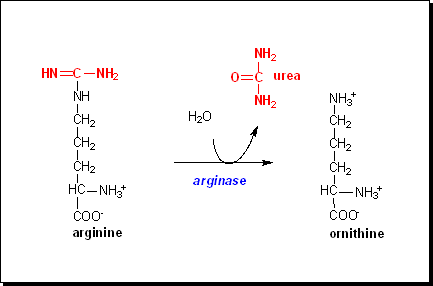
 One obvious
candidate would be the amino acid arginine; as shown on the left, hydrolysis
of the side chain of arginine yields urea.
One obvious
candidate would be the amino acid arginine; as shown on the left, hydrolysis
of the side chain of arginine yields urea. Having established that the source of half the nitrogen in urea comes from ammonium, by way of carbamyl phosphate, the next step in trying to elucidate the pathway of urea synthesis is to try to discover what might be the final intermediate before the formation of urea. In other words, what compound is likely to yield urea.
 One obvious
candidate would be the amino acid arginine; as shown on the left, hydrolysis
of the side chain of arginine yields urea.
One obvious
candidate would be the amino acid arginine; as shown on the left, hydrolysis
of the side chain of arginine yields urea.
If you incubate isolated hepatocytes with increasing concentrations of arginine, you will see that there is a steady increase in the formation of urea at low concentrations of arginine, levelling off as the enzyme becomes saturated.
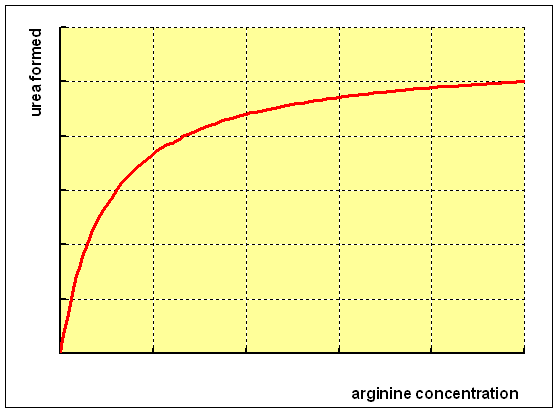
This suggests very strongly that the immediate precursor of urea is indeed arginine, and from the chemistry of the arginase reaction you would expect to form 1 mol of urea for each mol of arginine consumed.
In the simulation you will investigate the effect of adding three different concentrations of arginine to isolated liver cells incubated with a range of concentrations of ammonium. You will be given results for not only the amount of urea formed, but also the amount of ammonium remaining. Think about the results very carefully.
Can you explain your observations?