Sex
in model systems (HS)
In mammals
primary sex determination, whether the gonads develop as ovaries or testes,
depends on the presence of absence of a Y chromosome. Secondary sex
determination, the male/female phenotype of the rest of the body depends on the
presence or absence of hormones secreted by the testes. In fruit flies and
nematodes primary sex determination depends on the ratio of X chromosomes to
autosomes. X0 individuals develop as males even though no Y chromosome is
present. Neither animal possesses the equivalent of systematically acting sex
hormones. In Drosophila
sex determination is entirely cell autonomous (in somatic cells) and in C.
elegans only short range
cell-cell signalling is involved.
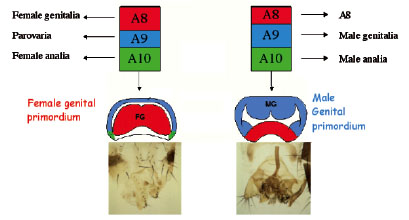
Drosophila male and female genitalia and analia
develop from the genital imaginal disc. Three abdominal segments give rise to
this disc, A8, A9 and A10. The A10 derived portion of the disc gives to the
analia of both sexes, A8 to female genitalia and A9 to male genitalia. In
females the growth of A8 is promoted at the expense of A9 and the reverse is
true in males.
To determine
whether to follow the female or male pathway depends on the activity of a series
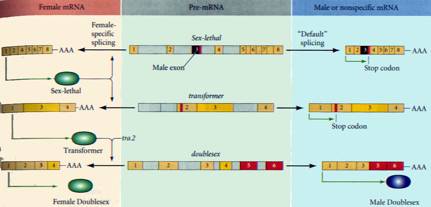
of sex determining genes in each cell. These include the RNA splicing
regulators Sxl, tra and tra2, the dsx and the ix loci. Sxl has two promoters. In early development one is
inactive and the state of the other depends on the relative activity of various
X-linked numerator and autosome-linked denominator genes. Numerator proteins
can bind to and activate the Sxl promoter except when bound to denominator
proteins. In XX individual which have equal numbers of copies of X and
autosomal genes sufficient numerator protein is available to activate the
promoter. The short transcript produced from this promoter is spliced to remove
an exon containing a pre-mature stop codon and full length Sxl protein is made.
Later, when
numerator and denominator proteins cease to be made, a second more 5Õ promoter
of the Sxl gene becomes active. This produces a longer transcript, for which
the splicing machinery requires Sxl protein to splice out the stop codon. In
males, because early synthesis of Sxl is not activated this splicing does not
occur and no active Sxl protein can be made.
Sxl also
regulates the splicing out of a stop codon containing exon in the tra gene. Its
presence in females and absence in males mean that tra protein is only
synthesised in females. Tra and tra2 regulate the splicing of dsx in females so
that exons 5 and six are excised – in males in the absence of tra/tra2
splicing between exons 3 and 5 removes exon four. Both splice forms produce
active proteins but the female-specific form forms a complex with the ix gene
product that promotes female-specific gene expression while the male form
activates male-specific patterns of expression.
While this
genetic pathway is used to determine the sex of somatic cells a somewhat
different combination of genes regulates the sex of germ cells - whether they
differentiate as sperm or eggs. The embryonic gonad is a separate organ from
the genital discs, which originates as an outgrowth of the mid-gut into which
germ cells migrate and coalesce with the somatic cells around 12 hours
post-fertilisation. As in mice, the germ cell development depends on signals
from the surrounding somatic cells. In both cases chromosomally male germ cell
with begin to develop as eggs when transplanted into ovaries and female germ
cells will begin to develop as spermatozoa when transplanted into testes. In
both mice and flies, however the subsequent development and proliferation of
transplanted germ cells becomes abnormal, in mice very few sperm generated from
transplanted XX germ cells survive in the adult mouse and in flies XY germ
cells form ovarian tumours. Clearly some germ cell-autonomous gene expression
is required for normal development. In flies, for example, there is a
requirement for sxl and other genes not involved in somatic cell sex
determination such as ovarian tumor (out) in female germ cell development.
Also as in mice
the genes at the top of the sex determination pathway are evolutionarily
labile. Just as Sry is found only in marsupials and eutherian mammals, sex-specific
expression of Sxl is confined to the Drosophila genus. House flies and medflies both use
a male determining factor on the Y chromsome to repress tra function.
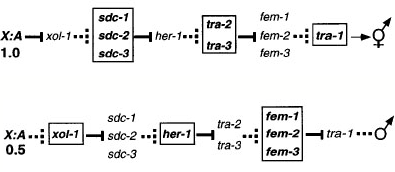
In C. elegans the two sexes are males and
hermaphrodites with the XX hermaphrodites being essentially females that
produce and store a few sperm at the beginning of adult life. The sex
determination pathway in C.elegans
is genetically complex. A gene called xol-1 responds to a numerator:denominator
signal and, through a series on negative regulators, this determines the
expression of a transcription factor called tra-1, which is off in males and on
in females.
One of the
downstream targets of tra-1 one is the male ray determinant mab-3 which shows homology
with the male-specific form of Drosophila dsx and the vertebrate gene DMRT1.
Recent evidence indicates that DMRT1 also acts as a downstream male
determinant. It is upregulated in the male genital ridge of mice, chicks and
alligators and deletions of DMRT1 in humans are associated with XY sex
reversal.
References
Gilbert 9th
ed Chapter 17 pp 543-552
Sex
determination gene and pathway evolution in nematodes (2003) Stothard P and
Pilgrim D Bioessays vol 25 pp221-231