ESTABLISHING THE DORSAL-VENTRAL AXIS OF THE DROSOPHILA EMBRYO
We have learned about how the basic segmentation pattern gets set up in the anterior-posterior axis (the segmentation genes). There is another dimension to the embryo - the dorsal-ventral axis. Within each segment, specific structures decorate the dorsal and ventral sides - in T2, wings are formed dorsally and legs ventrally. How does this pattern become established?
There is a set of about 20 genes that is responsible for DV axis formation. One of these, the dorsal (dsl)gene, encodes the primary morphogen. The others are there "merely" to ensure that Dorsal protein gets distributed in a gradient around the DV axis. In fact, Dorsal is expressed uniformly around the circumference of the cellular blastoderm, but is distributed unequally between the nucleus and the cytoplasm, being predominantly in the nucleus of cells that are destined to form the ventral side and in the cytoplasm of cells detined to be dorsal. Dorsal is a transcription factor so it must gain access to the nuclear DNA in order to exercise its function. Thus, it is a gradient of nuclear Dorsal protein that acts as the primary morphogen.
[Note that genes in Drosophila are named for the phenotypes that result when the gene in question is inactivated. When dsl is mutated the embryo does not develop any ventral structures - it is "dorsalized".]
Other genes in the group include cactus, toll, spätzle, easter, snake and gastrulation defective. Cactus binds to Dorsal in the cytoplasm and prevents it from going to the nucleus. Toll is a transmembrane tyrosine kinase receptor that is in the membranes of all cells around the embryo. When it is activated by binding to its extracellular ligand Spätzle, it sends a signal into the cell that causes Cactus to release Dorsal - possibly via a phosphorylation event. Toll is activated by Spätzle only on the future ventral side, so Dorsal is allowed to translocate into the nucleus only in ventral cells. Dorsal activates the ventralizing genes twist and snail in ventral cells and inhibits dorsalizing genes such as tolloid, decapentaplegic and zerknüllt, which become sequentially active in more dorsal parts of the embryo where Dorsal protein is more-and-more excluded from the nucleus.
What prevents Spätzle from binding to Toll in all cells, dorsal and ventral? Spätzle protein is present all through the perivitelline space (the gap between the eggshell and the embryo proper), but is only active on the ventral side. Spatzle is made as a precursor protein that is unable to bind its receptor Toll; in order to bind it must be cleaved by Easter, a serine protease (ie one that cuts the polypeptide backbone at serine residues). Why, then, is Spätzle cleaved only at the ventral side of the embryo? Easter is inactive as a protease until it is itself cleaved by another protease, Snake. Snake in turn must be activated through cleavage by Gastrulation defective. Thus activation of Toll on the ventral side relies on a cascade of proteolytic cleavage initiated by Gastrulation defective, which is embedded in the inside surface of the egg case at the ventral side. It follows that the DV asymmetry of the embryo is determined by a prior asymmetry in the egg case. The egg case is not part of the embryo but is made from the mother's follicle cells which surround the developing oocyte during oogenesis. DV asymmetry is pre-determined by the mother fly.
How does Gastrulation defective get localized to the ventral side of the eggshell? This is not completely understood but the early stages of its localization are being worked out. Towards the end of oogenesis, the oocyte nucleus becomes localized within the oocyte at the dorsal, anterior side. Here it transcribes mRNA for Gurken, a transmembrane protein, which is inserted into the oocyte plasma membrane at the anterior dorsal surface. The extracellular part of Gurken contains an EGF-like motif (EGF=epidermal growth factor) which activates the Drosophila EGF receptor (Torpedo, Top) in the mother's follicle cells at the dorsal side of the oocyte. This inhibits synthesis of certain genes (windbuetel, nudel, pipe) that are required to localize Gastrulation defective at the membrane, thus introducing DV asymmetry into the follicle cells. Remarkably, this asymmetry is then relayed back to the developing egg via the Dorsal group genes discussed above (see diagram below). Why such a complicated chain of events must be used is not known. However, it must be remembered that the eggcase itself has a function - it must protect the embryo from its environment (a soggy piece of banana perhaps) and has a specialized structure to enable it to fullfil this task. For example, there are two hollow appendages (rather like reindeer antlers) attached to the dorsal surface of the egg; these are thought to transfer air from above the surface of the substrate (aforementioned banana e.g.) to the embryo (read the minireview by L.Stevens, below). Maybe it is important that the asymmetries inherent in the egg and the embryo are correctly aligned with one another.
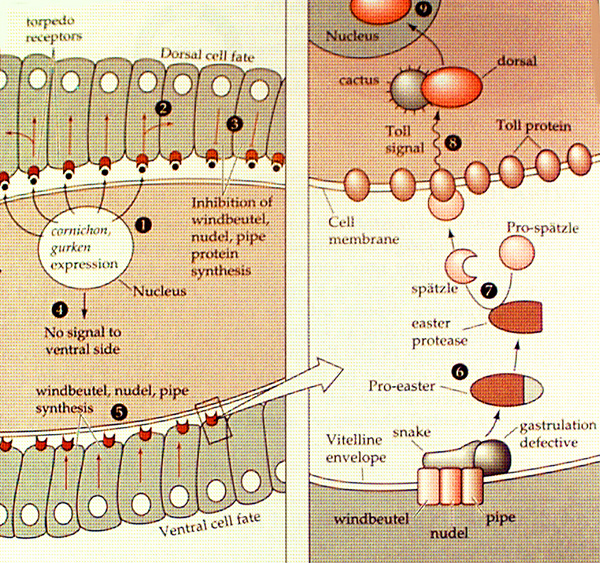 from Gilbert 4th edition, Chapter 15 p564
from Gilbert 4th edition, Chapter 15 p564
Note that the protease cascade that leads from Gastrulation defective through Snake, Easter, Spätzle to the Toll receptor has parallels in the protease cascade that is responsible for blood clotting in mammals. Furthermore, the signalling pathway downstream of Toll activation - which culminates in translocation of Dorsal protein to the cell nucleus - is preserved intact in mammalian cells. Dorsal is a member of the Rel family of transcription factors which includes NF-kappa-B (NF-kB). This factor binds to an enhancer (regulatory) sequence in the immunoglobulin (Ig) locus and activates Ig transcription in B-lymphocytes. Nomally NF-kB is complexed with an inhibitory protein I-kB (similar in sequence to Cactus) in the cytoplasm of the cell, preventing it from entering the nucleus and interacting with the Ig gene. Activation of the cell-surface interleukin-1 receptor (IL-1R) (similar in sequence to the Toll receptor) results in the release of NF-kB from I-kB and activation of Ig transcription.
1. Wolpert Chapter 5, p139-
2. Gilbert 6th edition Chapter 9, pp290-297 (5th edition Chapter 14, pp577-585)
3. St Johnston, D. (1993). Getting to the top. Current Biology 4, 54-56.
4. Roth, S. (1994). Proteolytic generation of a morphogen. Current Biology 4, 755-757.
5. Stevens, L. (1998).