|
Contact Info :
|
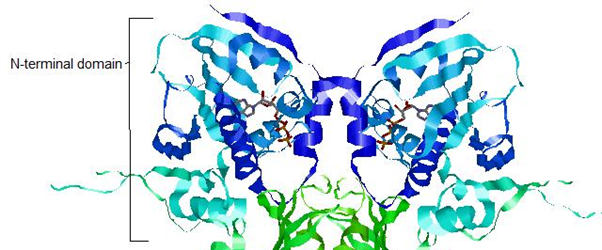
HSP90 - N Terminal Domain
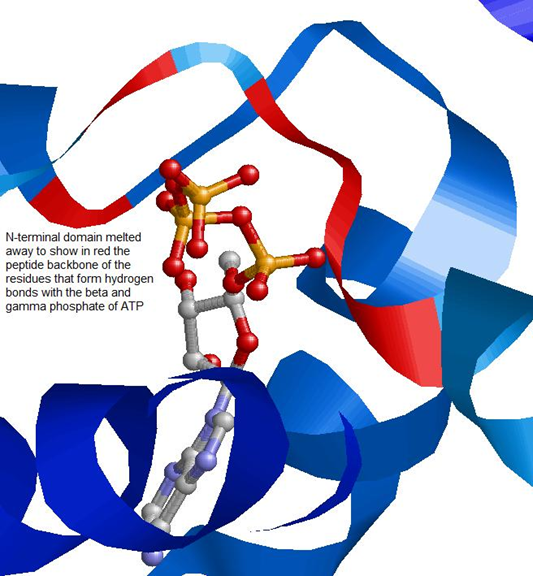
The N-terminal domain shows ATPase activity which is important in regulating the activity of Hsp90.
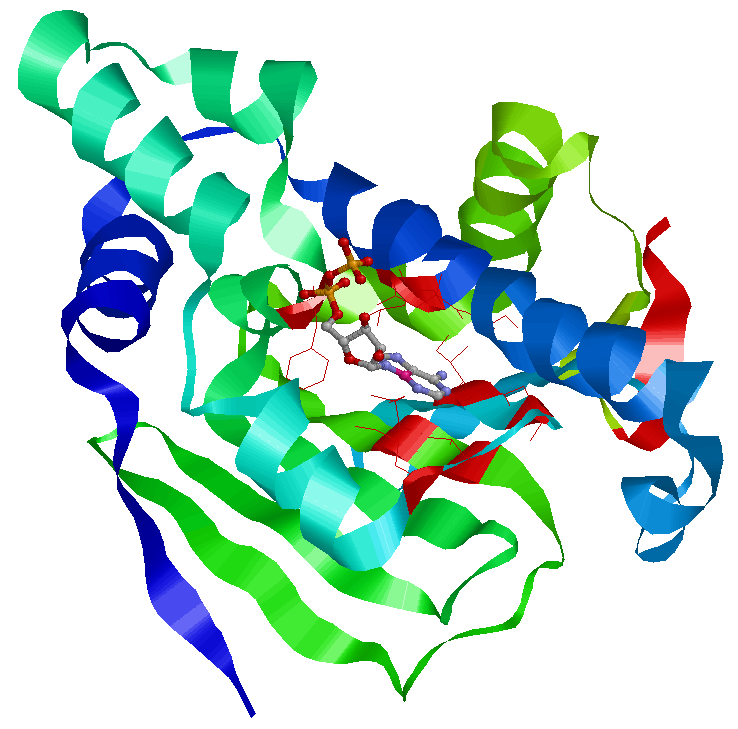
The residues in red make a pocket of hydrophobic and electrostatic interactions that hold the nucleotide in place. Hsp90 switches between two states according to whether it is bound to ADP or ATP.
The ATP binding domain has a lid structure that closes during ATP binding and opens when bound to ADP. This is shown below.
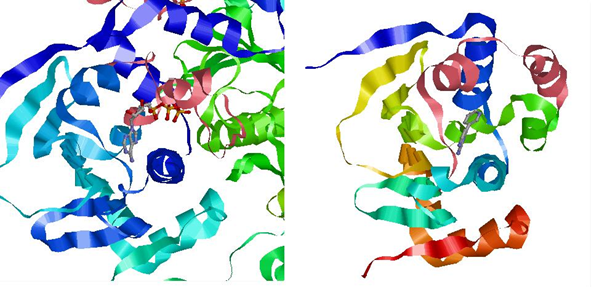
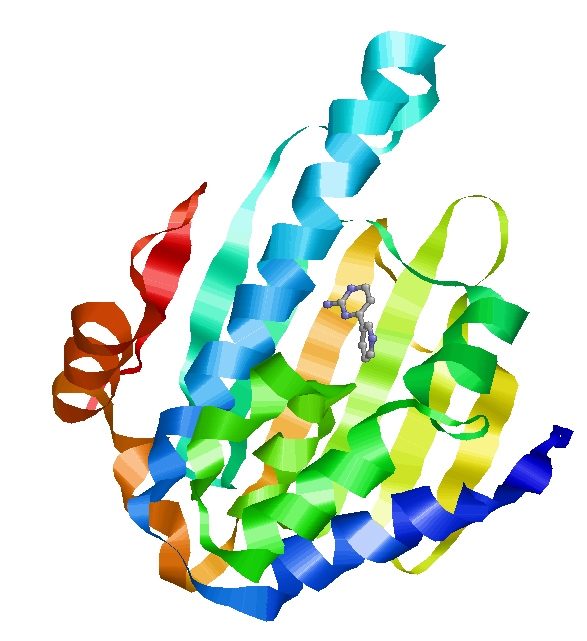
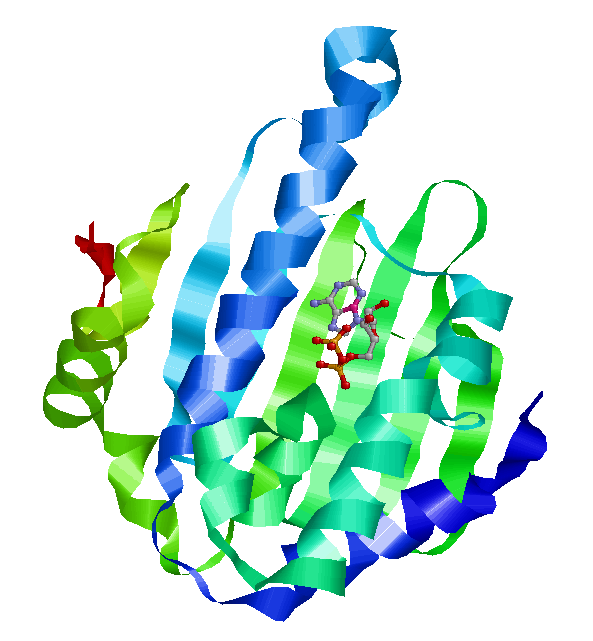
The lid is shown in pink. On the left is the lid in closed form, wrapped tightly around the nucleotide. On the right is the open form, with the lid further away from the nucleotide. Left: Crystal structure of N-terminal domain of Plasmodium falciparum Hsp90 (PF07_0029) bound to ADP (PDB). Right: 2XDK Homosapien N-terminal domain Crystal with inhibitor (PDB).
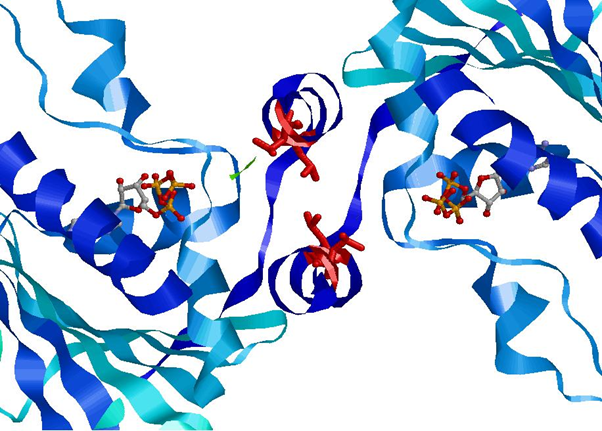
The movement of this lid into the closed position upon ATP binding exposes certain residues that react with the other N-terminal domain of the dimer.
The Leucine residues (Leu15 and Leu18) shown in red form hydrophobic interactions between the two N-terminal domains of the Hsp90 dimer once exposed by lid movement (Crystal Structure of an HSP90-SBA1 Closed Chaperone Complex (PDB)). This conformational change is responsible for the clamping of client protein by Hsp90, discussed later.
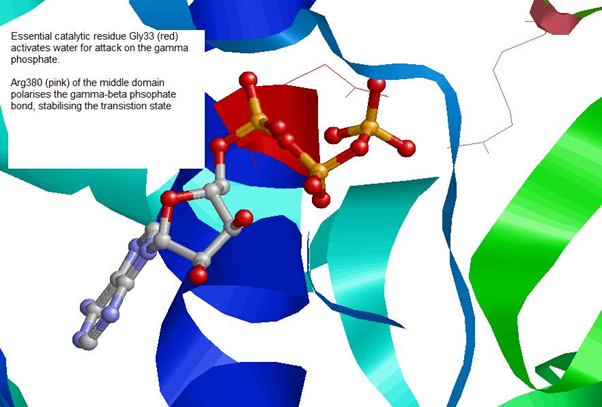
ATPase Catalytic ActivityImages of Crystal Structure of an HSP90-SBA1 Closed Chaperone Complex (PDB)
The ATPase domain of Hsp90 is highly conserved. Below the similarity between Homosapien and Plasmodium is shown. Homosapien N-terminal
Plasmodium N-terminal domain
|