Immune Regulation and Tumour Immunotherapy Group
Group Leader: Professor Sergio A Quezada
The Immune Regulation and Tumour Immunotherapy group at the UCL Cancer Institute aims to investigate the interplay between the immune system and cancer throughout tumour progression and immunotherapy. The use of our own immune system to specifically target cancerous cells has become a promising approach in the fight against cancer. However, anti-tumour immunity is tightly regulated by cellular and molecular circuits that prevent self and tumour destruction and significantly limit the efficacy of existing therapies.
CD4+ T cells play a key role in the regulation of immune responses to self and foreign antigens, differentiating into various subsets of helper and regulatory T cells and instructing the function of CD8+ T cells, NK cells and macrophages. Nonetheless, little is still known about the biology of tumour-reactive CD4+ T cells during tumour progression and cancer immunotherapy.
Our aim is to identify and target the most relevant cellular and molecular pathways restricting the activation of tumour-reactive lymphocytes, their access to the tumour site, and their activity within the tumour microenvironment. Moreover, we are interested in how the function and plasticity of tumour-reactive CD4+ T cells and the innate immune compartment is regulated by the tumour microenvironment and by immune co-inhibitory (e.g. CTLA-4 and PD-1) and co-stimulatory signals (e.g. GITR, OX40, CD27). In addition, we are interested in understanding how these regulatory circuits control the efficacy of cellular vaccination and adoptive cell transfer strategies and how can they be manipulated to induce potent anti-tumour immunity.
These studies will not only inform the basic understanding of the immune response to malignancies, but in the context of the UCL Cancer Institute, will be used as a platform for the development of novel translational strategies in the clinic.
Research Projects include:
- Mapping out the T Cell Landscape in Solid Tumours
The contribution of neoantigen reactive T cells to anti-cancer immunity has become increasingly well-established over the last decade. A key role for neoantigen-driven tumour recognition in humans is supported by the observations that i) patients with greater non-synonymous mutational burden (TMB) exhibit favourable clinical response to checkpoint blockade, ii) neoepitope-specific T cells can be detected in clinical samples, iii) adoptive T cell therapies that elicit radiographic responses contain neoantigen reactive T cell clones and iv) footprints of immune escape are enriched in tumours with an elevated neoepitope load. However, the mechanisms underpinning neoantigen-specific T cell responses remain poorly characterized. Therefore, understanding how the neoantigen-driven T cell response is orchestrated at the cellular and molecular level may optimise cell therapies, identify novel immunotherapy targets and define clinically relevant biomarkers.
Our team is working together with several clinical trials and biobanks including TRACERx (for lung cancer) and APADTER (renal cancer), DECIPHER (prostate) and Glioblastomas to understand the different T cell states and populations in these cancers and how TCR heterogeneity and T cell exhaustion correlates with poor clinical outcomes and to better understand mechanisms of response and resistance to immune checkpoint blockade.
- Understanding the Mechanisms of Action of Immunomodulatory Agents
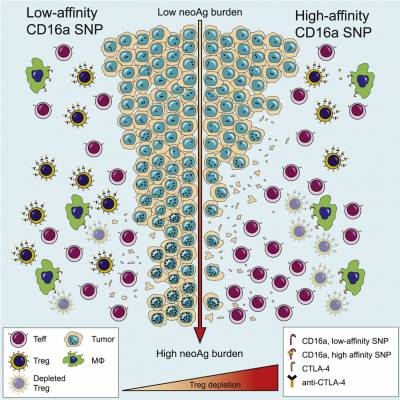
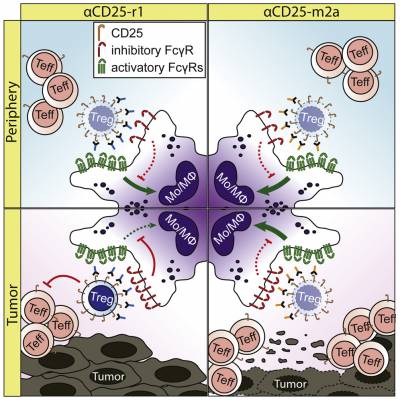
Our lab uses several mouse cancer models including lung, melanoma, colorectal and brain cancers to understand the mechanisms of action of immunomodulatory agents. When tumours are established, we administrate a variety of immunomodulatory agents and evaluate their tumour control efficacy and investigate the impact of these therapies on the immune landscape observed by high-dimensional flow cytometry. Using these models, we have unveiled the role of Fc/FcReceptor (FcR) interactions in the activity of immune modulatory monoclonal antibodies. We demonstrated a key role for antigen density and activating FcR in promoting depletion of tumour infiltrating regulatory T cells by anti-CTLA-4 antibodies (Arce Vargas et al., 2018) and a new anti-CD25 antibody developed by our team (Arce Vargas et al., 2017), currently under clinical evaluation (NCT04158583, www.clinicaltrials.gov).
Our team is now working to understand the role of FcγR-expressing cells on anti-CTLA-4 and anti-PD-1 treated tumours and further evaluate the function of FcγR engagement with agonistic antibodies.
- Cytotoxic Activity by Tumour Reactive CD4+ T Cells and their Potential Application in Tumour Immunotherapy
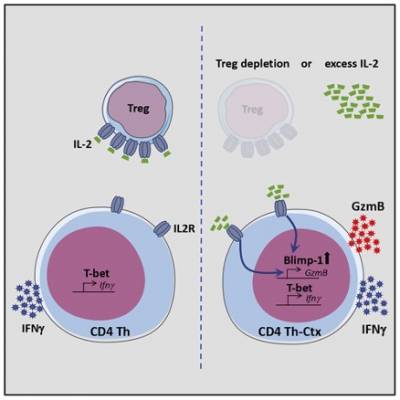
In addition to classical helper or regulatory activity, tumour-infiltrating CD4+ T cells can also acquire cytotoxic potential, marked by expression of Granzyme B. These cytotoxic CD4+ T cells directly kill tumour cells in an MHC-II-dependent manner, and promote rejection of established tumours in murine models of melanoma and sarcoma.
Recently, we showed that tumour-infiltrating CD4+ T cells acquire cytotoxic potential in response to IL-2 signalling, and require the transcription factor Blimp-1. Furthermore, differentiation of cytotoxic CD4+ T cells in tumours is limited by a high infiltration of suppressive regulatory T cells (Tregs), which act as a sink for IL-2. Currently, we are investigating the role of Blimp-1, and other transcription factors, in regulating CD4+ T cell differentiation and function in tumours.
Videos related to our research
Developing a new Treg-depleting antibody for cancer immunotherapy
Using monoclonal antibodies to target cancer
Publications
- Selected Publications
CD25-Treg-depleting antibodies preserving IL-2 signaling on effector T cells enhance effector activation and antitumor immunity.
Solomon, I, Amann, M., Goubier, A, Arce Vargas, F., Zervas, D, Qing, C, Henry JY, Ghorani, E, Akarca, A, Marafioti, T, Śledzińska, A, Werner Sunderland, M, Franz Demane, D, Ruth, JC, Georgiou, A, Salimu, J, Merchiers, P, Adrian Brown M, Flury, R, Eckmann, J, Murgia, C, Sam, J, Jacobsen, B, Marrer-Berger, E, Boetsch, C, Belli, S, Leibrock, L, Benz, J, Koll, H, Sutmuller, R, Peggs, KS, Quezada, SA .
Nat Cancer (2020). https://doi.org/10.1038/s43018-020-00133-0- Regulatory T Cells Restrain Interleukin-2- and Blimp-1-Dependent Acquisition of Cytotoxic Function by CD4+ T Cells.
Śledzińska A, Vila de Mucha M, Bergerhoff K, Hotblack A, Demane DF, Ghorani E, Akarca AU, Marzolini MAV, Solomon I, Vargas FA, Pule M, Ono M, Seddon B, Kassiotis G, Ariyan CE, Korn T, Marafioti T, Lord GM, Stauss H, Jenner RG, Peggs KS, Quezada SA.
Immunity. 2020 Jan 14;52(1):151-166.e6. doi: 10.1016/j.immuni.2019.12.007. Epub 2020 Jan 7 - The T cell differentiation landscape is shaped by tumour mutations in lung cancer.
Ghorani E, Reading JL, Henry JY, de Massy MR, Rosenthal R, Turati V, Joshi K, Furness AJS, Aissa AB, Saini SK, Ramskov S, Georgiou A, Sunderland MW, Wong YNS, De Mucha MV, Day W, Galvez-Cancino F, Becker PD, Uddin I, Ismail M, Ronel T, Woolston A, Jamal-Hanjani M, Veeriah S, Birkbak NJ, Wilson GA, Litchfield K, Conde L, Guerra-Assunção JA, Blighe K, Biswas D, Salgado R, Lund T, Al Bakir M, Moore DA, Hiley CT, Loi S, Sun Y, Yuan Y, AbdulJabbar K, Turajilic S, Herrero J, Enver T, Hadrup SR, Hackshaw A, Peggs KS, McGranahan N, Chain B, Swanton C, Quezada SA.
Nat Cancer. 2020 May;1(5):546-561. doi: 10.1038/s43018-020-0066-y. Epub 2020 May 22 - Spatial heterogeneity of the T cell receptor repertoire reflects the mutational landscape in lung cancer
Joshi K, Robert de Massy M, Ismail M, Reading JL, Uddin I, Woolston A, Hatipoglu E, Oakes T, Rosenthal R, Peacock T, Ronel T, Noursadeghi M, Turati V, Furness AJS, Georgiou A, Wong YNS, Ben Aissa A, Werner Sunderland M, Jamal-Hanjani M, Veeriah S, Birkbak NJ, Wilson GA, Hiley CT, Ghorani E, Guerra-Assunção JA, Herrero J, Enver T, Hadrup SR, Hackshaw A, Peggs KS, McGranahan N, Swanton C, Quezada SA, Chain B
Nat Med. 2019 Oct;25(10):1549-1559. doi: 10.1038/s41591-019-0592-2. Epub 2019 Oct 7. - Lost in Translation: Deciphering the Mechanism of Action of Anti-human CTLA-4.
Quezada SA, Peggs KS.
Clin Cancer Res. 2019 Feb 15;25(4):1130-1132. doi: 10.1158/1078-0432.CCR-18-2509. Epub 2018 Oct5 - The "Achilles' Heel" of Cancer and Its Implications for the Development of Novel Immunotherapeutic Strategies.
Joshi K, Chain BM, Peggs KS, Quezada SA.
Cold Spring Harb Perspect Med. 2018 Jan 2;8(1):a027086. doi: 10.1101/cshperspect.a027086. - Differential binding affinity of mutated peptides for MHC class I is a predictor of survival in advanced lung cancer and melanoma.
Ghorani E, Rosenthal R, McGranahan N, Reading JL, Lynch M, Peggs KS, Swanton C, Quezada SA
Ann Oncol. 2018 Jan 1;29(1):271-279. doi: 10.1093/annonc/mdx687. - Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies
Arce Vargas F, Furness AJS, Litchfield K, Joshi K, Rosenthal R, Ghorani E, Solomon I, Lesko MH, Ruef N, Roddie C, Henry JY, Spain L, Ben Aissa A, Georgiou A, Wong YNS, Smith M, Strauss D, Hayes A, Nicol D, O'Brien T, Mårtensson L, Ljungars A, Teige I, Frendéus B; TRACERx Melanoma; TRACERx Renal; TRACERx Lung consortia, Pule M, Marafioti T, Gore M, Larkin J, Turajlic S, Swanton C, Peggs KS, Quezada SA.
Cancer Cell. 2018 Apr 9;33(4):649-663.e4. doi: 10.1016/j.ccell.2018.02.010. Epub 2018 Mar 22. - The function and dysfunction of memory CD8+ T cells in tumor immunity
Reading JL, Gálvez-Cancino F, Swanton RC, Lladser A, Peggs K, Quezada SA
Immunol Rev. 2018 May;283(1):194-212. doi: 10.1111/imr.12657. - Urine-derived lymphocytes as a non-invasive measure of the bladder tumor immune microenvironment.
Wong YNS, Joshi K, Khetrapal P, Ismail M, Reading JL, Sunderland MW, Georgiou A, Furness AJS, Ben Aissa A, Ghorani E, Oakes T, Uddin I, Tan WS, Feber A, McGovern U, Swanton C, Freeman A, Marafioti T, Briggs TP, Kelly JD, Powles T, Peggs KS, Chain BM, Linch MD, Quezada SA
J Exp Med. 2018 Nov 5;215(11):2748-2759. doi: 10.1084/jem.20181003. Epub 2018 Sep 26 - Fc-Optimized Anti-CD25 Depletes Tumor-Infiltrating Regulatory T Cells and Synergizes with PD-1 Blockade to Eradicate Established Tumors
Vargas FA, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH, Rota EM, Dahan R, Georgiou A, Sledzinska A, Ben Aissa A, Franz D, Sunderland MW, Wong YNS, Henry JY, O'Brien T, Nicol D, Challacombe B, Beers SA, Turajlic S, Gore M, Larkin J, Swanton C, Chester KA, Pule M, Ravetch JV, Marafioti T, Peggs KS, Quezada SA
Immunity. 2017 Apr 18;46(4):577-586. doi: 10.1016/j.immuni.2017.03.013. Epub 2017 Apr 11.
See further publications.
 Close
Close