Hydrogen is the fuel used in most fuel cells (though others such as methanol and formic acid can be used too). It is the lightest element in the universe and is made of a single proton (positively charged) and a single electron (negatively charged) – hydrogen gas is made from two hydrogen atoms bound together, H2. Though over 75% of the universe’s mass is made from hydrogen, H2 gas doesn’t exist on earth and must be made from other sources containing hydrogen, mainly by reacting methane with steam.
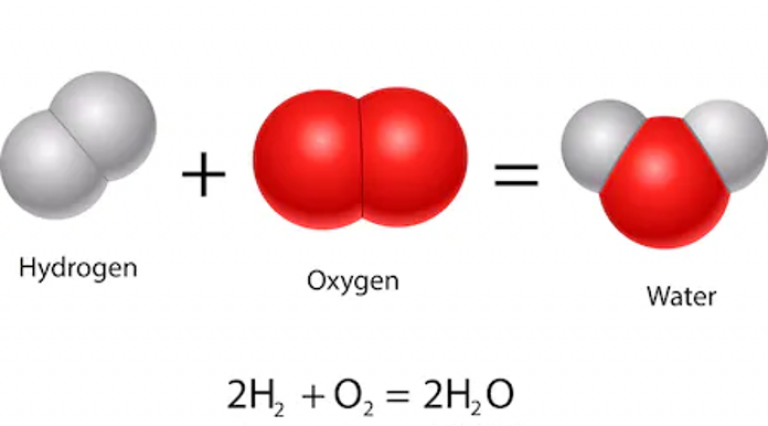
In a fuel cell, hydrogen is split into its protons and electrons and reacts with oxygen to form water. Because water is the only thing that comes out of the exhaust of a fuel cell, they are very clean. Hydrogen can also be made by doing the reverse reaction to a fuel cell using a device called an electrolyser. This uses electricity to split water (H2O) into hydrogen and oxygen. If renewable electricity is used for this process (rather than methane and steam), hydrogen can be a very clean fuel.
But why use electricity to make hydrogen just to make electricity later? As more renewable sources of electricity like wind and solar are added to the grid, it becomes difficult to spread out the supply and demand for power, as sometimes the wind isn’t blowing or the sun isn’t shining. If you make hydrogen when the wind is blowing, it can be stored and used later. Hydrogen can also be transported around the country to be used in other places from where the electricity is being made. In this way, hydrogen isn’t technically a fuel – you can think of it as a carrier of energy. Energy is put into splitting water, and then the hydrogen reacts in the fuel cell making energy and more water – a cycle. Most fuels that we think of such as petrol or natural gas contain energy from millions of years ago, locked up in the ground, until they are burned and that energy is lost for a very long time. The cycle for this is on the order of millions of years instead of a few seconds!
 Close
Close

