The Molecular NeuroPathobiology Laboratory
The research programme of the Molecular NeuroPathobiology Laboratory (MNP) is focussed on proving the hypothesis that impairment of the selectivity and/or efficiency of long-range communication in neurons caused by defects in membrane traffic constitute a major pathogenic mechanism in the nervous system. Indeed, the unique architecture and signalling capacity of neurons is maintained by trafficking events regulating the dynamics of intracellular organelles, such as signalling endosomes and mitochondria, and the spatio-temporal pattern of receptor stimulation by morphogens, neurotrophins and other growth factors. Axonal transport constitutes the backbone of this long-distance crosstalk, functionally connecting peripheral compartments, such as synapses, with events occurring in the soma and vice versa.
Our laboratory has provided crucial contributions to the field by defining the mechanism(s) responsible for the uptake and sorting of neurotrophin receptor complexes and virulence factors, and the coupling of these endocytic routes with the axonal retrograde transport pathway. We have demonstrated the essential role of cytoplasmic dynein and its adaptor proteins in this process, and provided a functional link between mutations in components of this transport pathway and neuronal dysfunctions. The translational potential of our findings have recently been confirmed by the discovery that these mutations are associated with a broad spectrum of disorders of the nervous system in human patients, including motor and sensory pathologies.
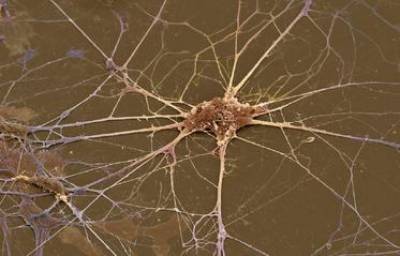
Figure 1. Primary spinal cord motor neuron in culture
Cellular Models: Our main model is the motor neuron, a type of neuron that functionally connect skeletal muscles with motor control centres located in the CNS. We isolate motor neurons either from embryonic spinal cord or derive them from mouse embryonic stem (ES) cells by in vitro differentiation. In addition, we also culture dorsal root ganglia, cortical, and hippocampal neurons.
Approaches: We have set up in our laboratory several techniques enabling us to study the composition, trafficking and signalling potential of signalling endosomes. We have performed imaging-based chemical and siRNA screens to test modulators of axonal transport and somatic sorting using ES-derived motor neurons, and established imaging protocols to monitor axonal transport both in vitro and in vivo using high resolution confocal and electron microscopy.
We strongly believe that only by understanding the basic principles of membrane trafficking and signalling will we be able to uncover the pathological changes responsible for many human disorders, including cancer. These fundamental processes might then be exploited to provide an earlier and more accurate diagnosis, translating into improved pharmacological treatments and more favourable prognosis.
Professor Giampietro Schiavo's UCL IRIS Profile
Current Projects
MNP Team Members
Publications
Collaborators
Grant Support
Picture and Movie Archive
Former MNP Team Members
Social MNP
Image Archive
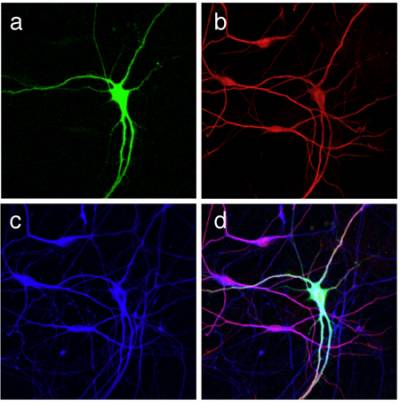
Figure 2 and 3. Differentiation of mouse ES cells into MNs. An optimised protocol allows the differentiation of primary MNs from a mouse ES cell line. MN specification is determined by the addition of retinoic acid and sonic hedgehog, whilst MN maturation is achieved in the presence of neurotrophins. ES cell-derived MNs are characterized by an HB9-driven GFP staining (green) and are positive for the bIII isoform of tubulin (red), and for the dendritic marker MAP2 (blue).
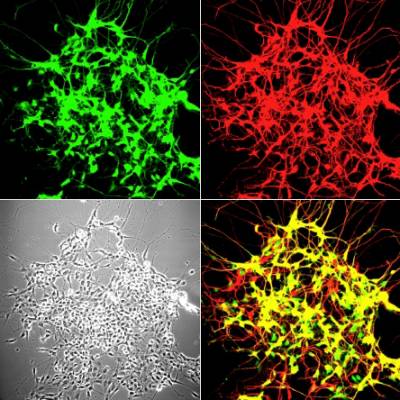
Figure 2 and 3. Differentiation of mouse ES cells into MNs. An optimised protocol allows the differentiation of primary MNs from a mouse ES cell line. MN specification is determined by the addition of retinoic acid and sonic hedgehog, whilst MN maturation is achieved in the presence of neurotrophins. ES cell-derived MNs are characterized by an HB9-driven GFP staining (green) and are positive for the bIII isoform of tubulin (red), and for the dendritic marker MAP2 (blue).
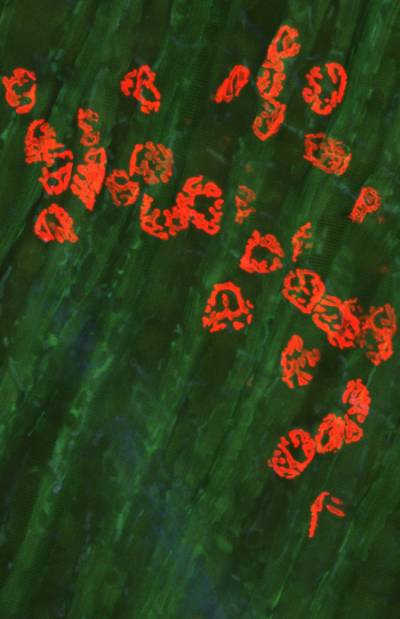
Figure 4 and 5. Mouse neuromuscular junctions (NMJ). In Figure 4, a post-synaptic marker (alpha-bungarotoxin - BTx; in red) highlight a group of NMJs. Figure 5 shows a close up of a NMJ and its axonal shaft stained with an axonal retrograde transport probe (in red) and BTx (in green).
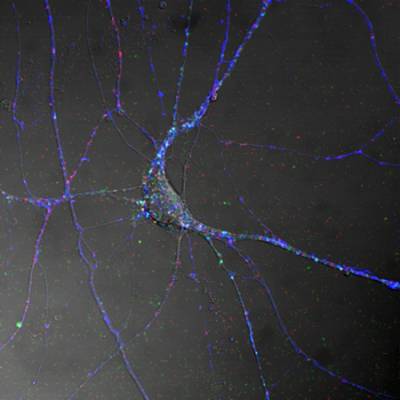
Figure 1. Spinal cord motor neurons control muscle contraction by electrical output signals. This figure shows the distribution of TeNT HC (blue), the Neurotrophin receptor p75NTR (red) and BDNF (green) in differentiated MN in culture.
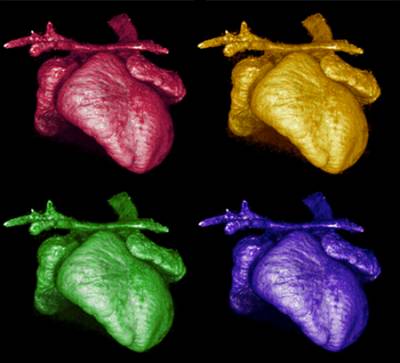
Figure 6. 3D-reconstruction by microtomography of the heart of a Kidins220/ARMS-/- embryo. Note the dilated atria and the altered outflow tract. Images collected and analysed by I.R. Orriss (UCL Institute of Ophthalmology), A. Weston and L. Collinson (Electron Microscopy Facility, LRI).
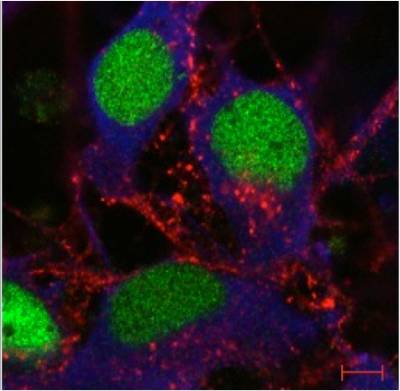
Figure 8. Rab7 and peripherin are co-expressed in sensory neurons. Peripherin displays a filamentous distribution in the axonal network of embryonic dorsal root ganglia (DRGs) in culture (in green), whereas Rab7 shows a very intense punctate staining in the soma and in the network. At close inspection, Rab7-positive organelles are often in close apposition to peripherin filaments. Scale bars, 10 µm.
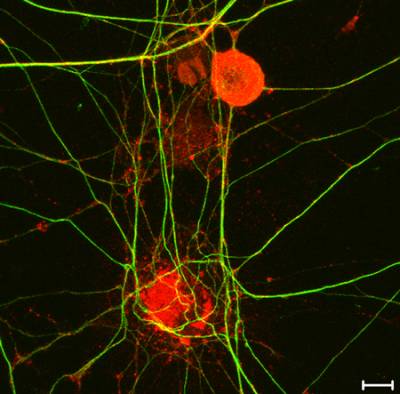
Figure 9. High-resolution mapping of axonal signalling endosomes. Confocal image of embryonic stem cells-derived motor neurons incubated with HCT conjugated to monocrystalline iron oxide nanoparticles (MIONs) for 1 h at 37˚C. HB9 motor neuron-specific promoter is depicted in green, the HA tag of TeNT HCT in red and bIII-tubulin in blue. Scale bar: 5 μm.
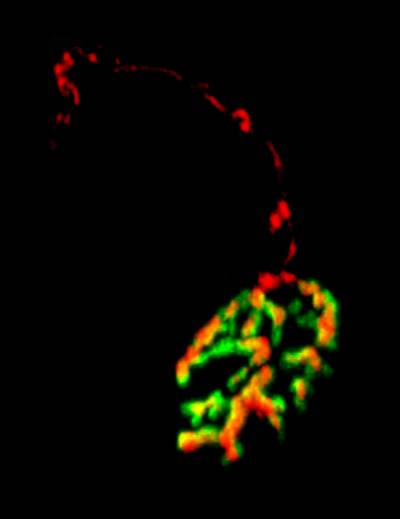
Figure 4 and 5. Mouse neuromuscular junctions (NMJ). In Figure 4, a post-synaptic marker (alpha-bungarotoxin - BTx; in red) highlight a group of NMJs. Figure 5 shows a close up of a NMJ and its axonal shaft stained with an axonal retrograde transport probe (in red) and BTx (in green).
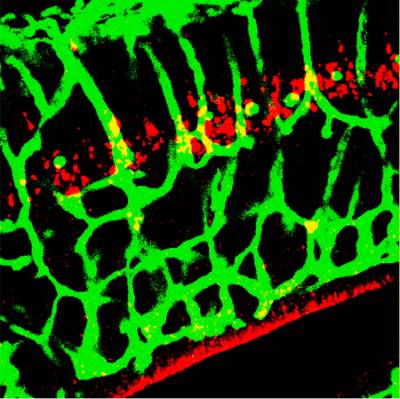
Figure 7. Kidins220/ARMS is required for capillary plexus development in the brain. A section of E16.5 Kidins220/ARMS-/- brain was stained with an endothelial cell marker (in green) and for active caspase-3 (in red).
 Close
Close

