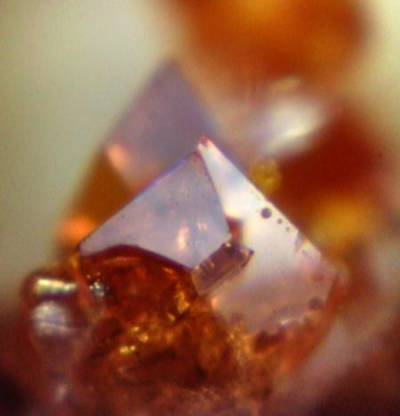
Orange crystals
20 May 2013

As well as being pretty, crystals tell us something profound: their shapes reflect the way they are structured on the tiniest of scales. The orderly arrangement of their atoms in a neat lattice scales up to produce crystals' simple, geometric shapes, making their appearance a peep-hole into the world of atoms and molecules.
Crystals can have extremely simple chemistry: diamond, for instance, is made only of carbon atoms, tightly bound together in a repeating pattern. Others are more complex, including the acetylferrocene crystal pictured here, which is made up of iron, carbon, hydrogen and oxygen. But they all share similar, simple, ordered structures.
This crystal of acetylferrocene was grown as part of an undergraduate course in UCL's Department of Chemistry. However, it has a number of uses in industry. In particular, it is used as an additive in rocket fuel, as it helps increase the propellant's rate of burning.
Credit: Andrea Sella (UCL Chemistry)
Copyright
- This image can be reproduced freely providing the source is credited.
 Close
Close