Heat treatment may make chemotherapy more effective
5 January 2021
Heating up cancer cells while targeting them with chemotherapy is a highly effective way of killing them, according to a new study led by UCL researchers.
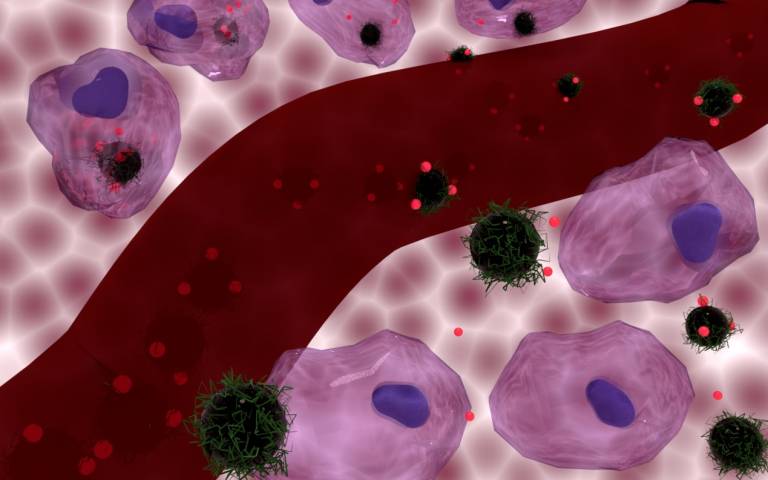
The study, published in the Journal of Materials Chemistry B, found that “loading” a chemotherapy drug on to tiny magnetic particles that can heat up the cancer cells at the same time as delivering the drug to them was up to 34% more effective at destroying the cancer cells than the chemotherapy drug without added heat.
The magnetic iron oxide nanoparticles that carry the chemotherapy drug shed heat when exposed to an alternating magnetic field. This means that, once the nanoparticles have accumulated in the tumour area, an alternating magnetic field can be applied from outside the body, allowing heat and chemotherapy to be delivered simultaneously.
The effects of the two treatments were synergistic – that is, each treatment enhanced the effectiveness of the other, meaning they were more potent when combined than when separate. The study was carried out on cells in a lab and further research is needed ahead of clinical trials involving patients.
Senior author Professor Nguyen T. K. Thanh (Biophysics Group, UCL Physics & Astronomy) said: “Our study shows the enormous potential of combining chemotherapy with heat treatment delivered via magnetic nanoparticles.
“While this combination of therapy is already approved for the treatment of fast-growing glioblastomas, our results suggest it has potential to be used more widely as a broad anti-cancer therapy.
“This therapy also has potential to reduce the side effects of chemotherapy, by ensuring it is more highly targeted on cancer cells rather than healthy tissue. This needs to be explored in further pre-clinical tests.”
In the study, researchers combined the magnetic nanoparticles with a commonly used chemotherapy drug, doxorubicin, and compared the effects of this composite in various scenarios on human breast cancer cells, glioblastoma (brain cancer) cells, and mouse prostate cancer cells.
In the most successful scenario, they found that heat and doxorubicin together killed 98% of brain cancer cells after 48 hours, when doxorubicin without heat killed 73%. Meanwhile, for the breast cancer cells, 89% were killed by heat and doxorubicin together, while 77% were killed after 48 hours by doxorubicin alone.
Cancer cells are more susceptible to heat than healthy cells - they undergo a slow death (apoptosis) once the temperature reaches 42 degrees Celsius, whereas healthy cells are able to withstand temperatures up to 45 degrees Celsius.
The researchers found that heating cancer cells by only a few degrees, to 40 degrees Celsius, enhanced the effectiveness of the chemotherapy, meaning the treatment could be effective with lower doses of nanoparticles.
They found the combination of therapies was most effective when the nanoparticles were absorbed, or internalized, by the cancer cells, but they found the chemotherapy was also enhanced when the nanoparticles shed heat while remaining outside the cancer cells (which would be an easier form of treatment to deliver). However, the effects at lower temperatures only occurred when the iron oxide nanoparticles were internalized or tightly deposited on to the surface of the cancer cells.
The nanoparticles also have a polymer coating that prevents the chemotherapy drug from leaching out into healthy tissue. The coating is heat and pH-sensitive, and is designed to release the drug when temperature rises and the nanoparticles are internalized within tiny pockets in cells called “lysosomes”, which have a lower pH than the rest of the cell medium. This intracellular delivery of the drug was particularly effective for the mouse prostate cancer cells, which showed superior and synergetic cell death effect, especially when the temperature reached 42°C.
Co-author Dr Olivier Sandre, of the University of Bordeaux, said: “Since heat can be generated through the alternating magnetic field, the release of the drug can be highly localised to cancer cells, potentially reducing side effects.”
Researchers received funding from the Engineering and Physical Sciences Research Council (EPSRC), the Asian Office of Aerospace Research and Development (AOARD), the European Cooperation in Science and Technology (COST), UCL, the University of Bordeaux, and collaborated with Resonant Circuits Limited.
Links
- Full paper
- Professor Nguyen T.K. Thanh’s academic profile
- Biophysics Group
- UCL Physics & Astronomy
- UCL Mathematical & Physical Sciences
- University of Bordeaux
Image
- An artist’s conception of the doxorubicin-loaded nanocomposite carriers being internalized by cells (at the top) and remaining outside cells (at the bottom), with a blood vessel at the centre. Credit: Journal of Materials Chemistry B / Nguyen T. K. Thanh / Florian Aubrit/ Olivier Sandre/ Lilin Wang.
Media contact
Mark Greaves
Email: m.greaves [at] ucl.ac.uk
 Close
Close

