Respiratory motion during PET acquisition leads to blurring in resulting images and quantification underestimation. Areas in the upper abdomen and thorax are adversely affected, especially lung/liver/pancreas lesions and the heart. In the case of lesions, PET images can provide information for monitoring of tumor response to therapy, diagnosis of malignancy via a standard uptake value (SUV) and calculation of lesion volumes for radiation planning, all of which are adversely affected by respiratory motion.
We have several projects running related to respiratory motion detection and correction for PET/CT and PET/MR, with the 2 main projects listed below. Our research to estimate respiratory motion directly from the PET data is listed on our image reconstruction page [LINK]. We have also just started a project for CBCT in radio-therapy.
Data-driven extraction of a respiratory signal from raw PET data
The first step in correcting for respiratory motion is to obtain a signal related to the respiration. With this signal, the data can for instance be binned ("gated") into (nearly) motion free data-sets prior to reconstruction by selecting the detected events with respect to the breathing phase. On current scanners the respiratory signal required to do so is obtained from an external device, whereas with data-driven (DD) methods it is directly obtained from the raw PET data, therefore avoiding the use of external equipment, which is expensive, needs prior setup and can cause patient discomfort.
Many DD methods have been developed over the years but we concentrate on using Principal Component Analysis (PCA) on dynamic PET sinograms of low spatial resolution [1]. This method is easy to implement and fast and has been shown to perform well compared to other methods in a preliminary study [2]. We are performing further evaluations, also in collaboration with the PET Center of Yale University http://petcenter.yale.edu/
 |
 |
However, many DD methods including PCA provide signals whose relationship with the physical motion is determined up to an arbitrary sign. This could cause inaccurate
motion correction especially with multiple bed positions, where consistency between adjacent beds is needed, and attenuation correction artifacts in PET/CT. We proposed new methods and compared them to a published registration-based method on FDG oncology patient data [3].
Clinically practical respiratory motion correction in simultaneous PET/MR
The recent advent of PET/MR scanners allows us to exploit the simultaneity of the modalities by using high spatial resolution and high contrast MR images to track respiratory motion and use it to correct PET data, without increasing patient scan times and radiation exposure. In collaboration with Dr David Atkinson (UCL Centre for Medical Imaging [LINK TO https://www.ucl.ac.uk/medical-imaging]), we proposed a practical respiratory motion correction regime that requires no external hardware to provide a respiratory signal and no change to the clinical protocol except a short extra MR sequence run after the clinical scan to provide a patient-specific motion model. The approach is anatomically general, applicable to any type of thorax/abdomen related motion caused by respiration e.g. lung, liver, pancreatic lesions and cardiac data.
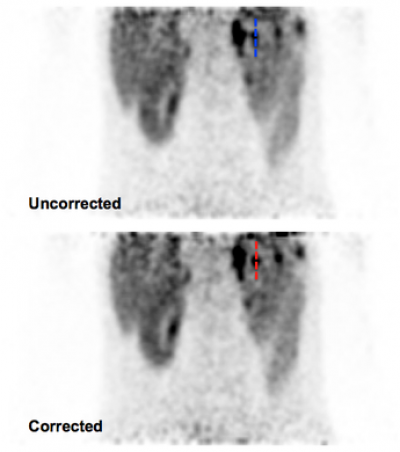
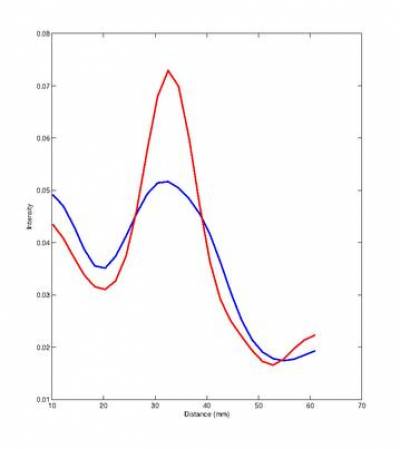
The method [4] works by extracting a respiratory signal from the PET data (see also previous section), using this PET-derived signal to bin PET and MR data, constructing a motion model from the binned MR data and using this data to correct PET data for respiratory motion. We have validated our PET-derived respiratory signal by comparison with an absolute measure of diaphragmatic displacement via in MR pencil-beam navigator. Motion-corrected images were compared to non-corrected images qualitatively, and quantitatively with line profiles and change in SUV through regions of high tracer uptake (see Fig. 2).
Current work focuses on building a continuous motion model from 1 min worth of dynamic MR data. This enables to extrapolate motion information even if the respiratory pattern during the PET or MRAC was not observed during the dynamic MR sequence, enabling use all of the PET data and avoiding artefacts when the MRAC was acquired at an atypical breathing stage.
References:
[1] Thielemans K, Rathore S, Engbrant F, Razifar P, "Device-less gating for PET/CT using PCA", Conf. Rec. IEEE NSS-MIC, Valencia, Spain (2011). http://dx.doi.org/10.1109/nssmic.2011.6153742
[2] Thielemans K, Schleyer P, Marsden PK, Manjeshwar RM, Wollenweber SD, Ganin A, "Comparison of Different Methods for Data-driven Respiratory Gating of PET Data", proc. IEEE MIC 2013, Seoul, Korea http://dx.doi.org/10.1109/nssmic.2013.6829055
[3] Bertolli O, Arridge S, Stearns CW, Wollenweber SD, Hutton BF, Thielemans K, "Sign determination methods for the respiratory signal in data-driven PET gating", IEEE Medical Imaging Conference, November 2015, San Diego, CA, USA.
[4] Manber R, Thielemans K, Hutton B, Barnes A, Ourselin S, Arridge S, O'Meara C, Wan S, Atkinson D (2015). "Practical PET Respiratory Motion Correction in Clinical PET/MR". J Nucl Med, Jun;56(6):890-6. http://dx.doi.org/10.2967/jnumed.114.151779
 Close
Close

