New publication in Nature Physics for Mao and Banerjee Labs
12 August 2019
This new publication reveals tissue fluidity promotes epithelial wound healing. They used the developing wing of the fruit fly, Drosophila, to understand how cells behave during wound healing, and find that reducing mechanical tension in cells.
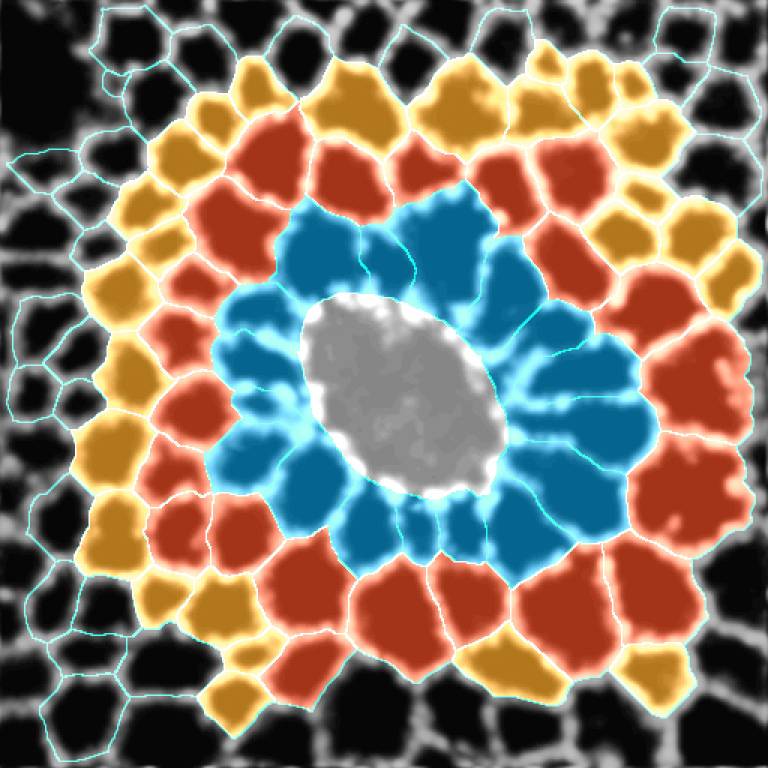
Abstract:
The collective behaviour of cells in epithelial tissues is dependent on their mechanical properties. However, the contribution of tissue mechanics to wound healing in vivo remains poorly understood. Here, we investigate the relationship between tissue mechanics and wound healing in live Drosophila wing imaginal discs and show that by tuning epithelial cell junctional tension, we can systematically alter the rate of wound healing. Coincident with the contraction of an actomyosin purse string, we observe cells flowing past each other at the wound edge by intercalating, reminiscent of molecules in a fluid, resulting in seamless wound closure. Using a cell-based physical model, we predict that a reduction in junctional tension fluidizes the tissue through an increase in intercalation rate and corresponding reduction in bulk viscosity, in the manner of an unjamming transition. The resultant fluidization of the tissue accelerates wound healing. Accordingly, when we experimentally reduce tissue tension in wing discs, the intercalation rate increases and wounds repair in less time and find that reducing mechanical tension in cells accelerates wound healing’.
Links
- Research paper in Nature Physics
- Yanlan Mao's academic profile
- Shiladitya Banerjee's academic profile
- UCL MRC Laboratory for Molecular Cell Biology
- UCL Physics & Astronomy
- UCL Institute for the Physics of Living Systems
 Close
Close

