Introduction
In order to combat the pathological polymers which lead to serpinopathic diseases (including antitrypsin deficiency as well as Alzheimer’s) we have developed in the last 2 decades an expansive tool box of techniques and protocols aimed to expedite translation to the clinic, employing structural, molecular and cell-based studies, alongside work on animal models (C. elegans) and with an extensive use of target-based and phenotypic drug screening.
We believe that a multi-disciplinary approach represents (and even more in the future) the right approach to investigate, characterise and tackle a genetic disorder like alpha-1 antitrypsin deficiency (AATD).
In addition, another arm of our group is involved in the development of drugs/chemicals for human and animal health as well as for pest control to eradicate human tropical diseases involving nematodes, ticks and mosquitos or other threats to human, animal and crop health by specifically targeting ion channels or receptors using INVAPP (Invertebrate Phenotyping Platform) and Electrophysiology.
Biochemical and Biophysical Techniques
Point mutations can cause serpins to be retained as ordered polymers and lead to disease by two different but related events.
Accumulation of mutated proteins within the cells could drive the formation of polymers or aggregates that results in toxicity and bring, through different cellular mechanisms, cell death. The intracellular retention, at the same time, could bring to a loss of function due to a lack of secretion of the protein and the consequent inability to carry out its activity where needed. AATD represents a classical example for this process.
Alpha-1 antitrypsin (AAT) is mainly synthesized within hepatocytes and with a level of 0.9 to 1.75 mg per millilitre, represents one of the most abundant plasmatic proteins (Strnad et al, N Engl J Med 2020; 382:1443-1455). AAT is a protease inhibitor targeting neutrophil elastase and its role is mainly related to prevent the destruction of tissue, particularly in the lung, from elastase activity. Due to point mutations, AAT could be affected by conformational changes that lead to its intracellular accumulation as polymers with a consequent strong reduction in secretion (loss of function) and, on the other side, to a gain of toxicity related to the accumulation of such polymers as inclusion bodies within the vesicle of endoplasmic reticulum.
AATD is a genetic disorder characterized by pulmonary disease, especially emphysema and bronchiectasis, and hepatic disease.
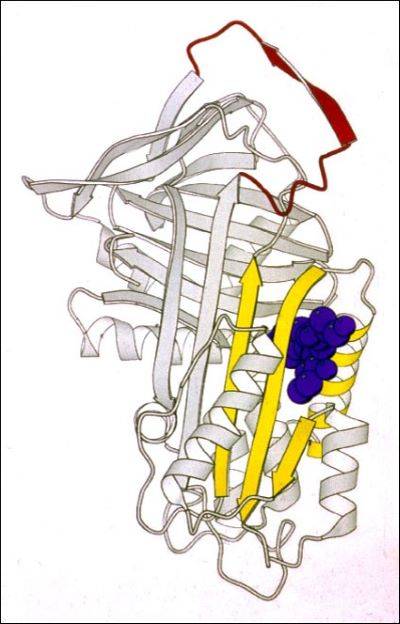
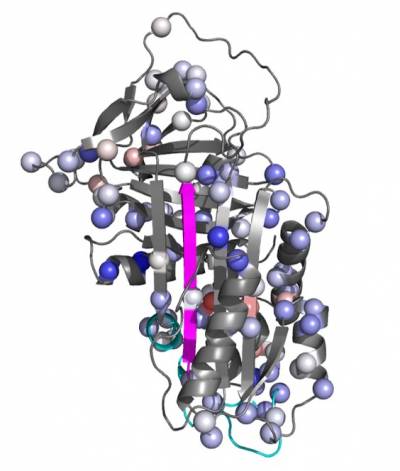
In our lab we use a wide range of biophysical techniques to investigate all the molecular details associated with the mechanism of folding and polymerisation of different mutants of AAT. In particular, we are able to produce high amount of recombinant AAT that will be used to deepen our understanding of the structural and molecular mechanisms that drive mutated AAT to form polymers. All these data are very useful for the understanding of the pathway of polymerisation and the intermolecular linkage employed in order to allow the rational design of small molecules to block AAT polymerization and treat AATD.
Here below a list of the techniques present in the lab:
Protein Purification, Differential Centrifugation, Enzyme/Protein assays (ELISA, polymerisation, protease inhibition, Thermal denaturation, Refolding), Enzyme Kinetics, Proteolysis, Protein Characterization, Antibody epitope mapping, Western blotting, Electrophoresis, Circular dichroism, Protein crystallography, Molecular Modelling, Molecular Biology, Mutagenesis, Recombinant Expression, Spectroscopy (EPR, ESR, Fluorescence), Mass Spectrometry, NMR and CryoEM.
Cell Biology
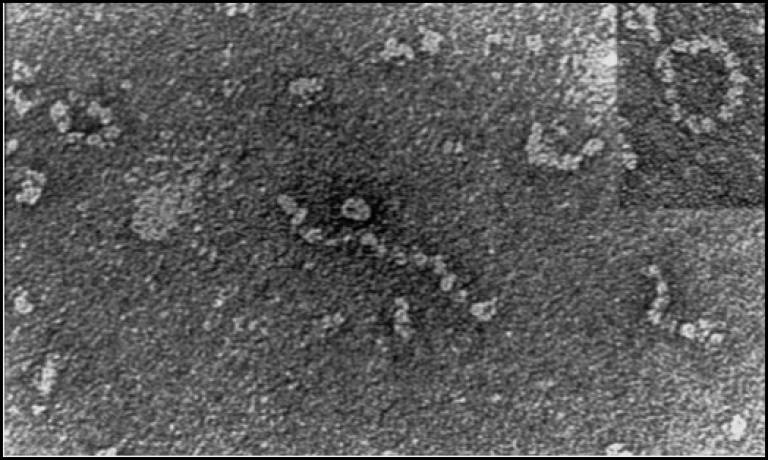
The Z mutation destabilises the native α1-AT and causes the formation of aberrant polymers that accumulate within the endoplasmic reticulum (ER) of hepatocytes, giving rise to inclusion bodies that are the main histological feature of α1-ATD (Lomas, DA, et al, Nature, 1992; 357: 605-607; Lomas, DA, et al, J. Hep, 2016; 65 (2): 413-424).
Approximately 70% of Z α1-antitrypsin is degraded within hepatocytes by ER associated degradation (ERAD). 15% folds effectively and is secreted, whilst the remainder self-associates to form polymers. These are in part degraded by autophagy with insoluble long-chain polymers trapped within the inclusion bodies, while at least part of the soluble, short-chain polymers have been shown to enter transport vesicles for secretion. Suggesting that polymers secreted from hepatocytes can contribute to circulating polymers. (Fra A. et al, ERJ, 2016; 47: 1005-1009). Misfolded proteins within the ER lumen usually trigger adaptive measures termed the unfolded protein response (UPR). However, the structurally ordered nature of the Z α1-antitrypsin polymers does not lead to UPR activation. Instead Z α1-antitrypsin expression activates NFκB by a calcium-mediated pathway that is independent of the UPR, which we have termed the ‘ordered protein response’ (Ordóñez, A. et al, Hepatology, 2013; 57: 2049-2060; Davies, MJ, et al, J Biol Chem, 2009; 284: 18202-18209).
In order to determine the intracellular dynamics and fate of all antitrypsin conformers and to evaluate the efficacy and toxicity of small molecules to block intracellular polymerisation, we have developed a wide range of techniques that allow us to investigate meticulously the various aspects of the accumulation and secretion of AAT in cells.
In our laboratory we have the opportunity to work with a large and varied bank of cells. Hepa 1.6, HepG2 and the most famous HeLa are used to evaluate the dynamics and intracellular mechanisms to which AAT is subject. These cells, suitably transfected, allow us in a short time to have a clear picture of the behaviors that new variants of AAT have inside the cell or, on the other side, how specific mechanisms can be modified using small molecules or drugs that act specifically on cellular pathways (e.g. proteasomal degradation, autophagy, synthesis). For large-scale production of AAT for further purification and analysis with biophysical techniques, we use Hek293T and CHO cells suitably engineered for the inducible expression of AAT (TET ON system). Our bank is completed with the presence of induced pluripotent stem cells (iPSCs) – derived hepatocytes obtained from a patient homozygous for the Z variant of AAT. More recently, we have started a collaboration to expand our bank with organoids always derived from AATD patients.
Another crucial and quite unique tool we have in our lab is represented by a bank of monoclonal antibodies, produced and developed in our laboratory, able recognize specific structural and conformational aspects of AAT polymers and some of their conformational intermediates. These antibodies are mainly used to dissect the conformational intermediates characterizing all the steps in the folding, accumulation and secretion of this protein. They are used, as whole or just as Fabs (Fragment antigen-binding), both in the biochemical (Pulse chase, Western Blot, Immunoprecipitation and Immunofluorescence) and biophysical (Cryo-EM, Crystallography, NMR) analysis.
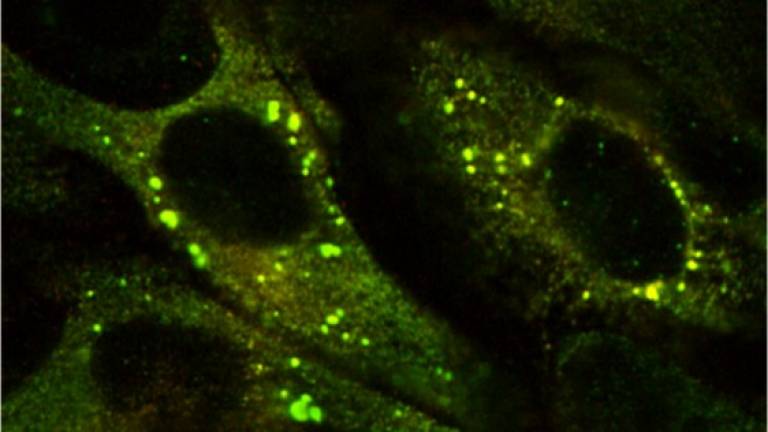
Ordóñez, Hep 2013 57(5):10
Inclusions of mutant Z a1AT in the ER
Genetic Model Organism and Automated Screening
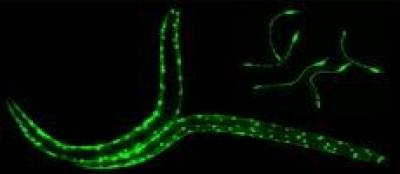
The powerful genetic toolkit of C.elegans, its rapid life cycle, transparency and low-cost maintenance makes it well suited for basic studies on the mechanisms underlying neurodegenerate and neuromuscular disorders. C.elegans is the only organism for which there is a complete nervous system wiring diagram.
Targeted expression of antitrypsin in the muscle cells (or intestinal cells, O’Reilly et al. Int J Biochem Cell Biol. 2014; 47: 109–112. ) of C. elegans serves as a model of α1-antitrypsin-deficiency and can be used as a tool for identifying genetic modifiers and small molecule drugs. High-throughput automated phenotypic drug screening of these compounds is achieved using the in-house developed Invertebrate Automated Phenotyping Platform (INVAPP) where machine vision, statistical imaging and tracking approaches enable the automated characterisation of nematode movement.
Automated measurement of nematode swimming (thrashing) in 96-well plates using a covariance-based statistical approach enables motility measurement suitable for use in high-throughput drug/chemical screens. The device (Wormwatcher) analyses movement by simultaneously recording movies of worms movement in all wells of a 96-well plate. The movie is analysed by generating a covariance matrix for each individual well showing the similarity between frames tx and ty. The time dependence of similarity between pairs of images is used to estimate the time interval between rhythmic worm movements, and hence the thrashing rate.
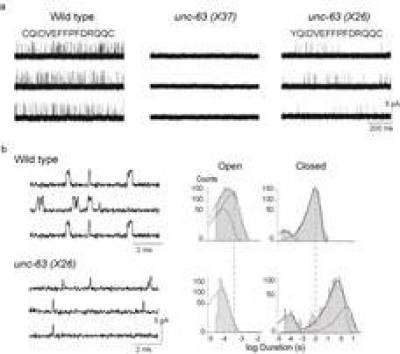
Ion Channel and Nicotinic Acetylcholine Receptor Electrophysiology
Ion channels are proteins embedded in the cell membrane which control the bidirectional movement of charged species called ions across the impermeable cell membrane. Ion channels are highly selective and can be grouped according to their structure, either as members of the voltage-gated or ligand-gated ion channel families.
The voltage- and neurotransmitter-gated ion channels i.e. ionotropic receptors such as Nicotinic Acetylcholine Receptors (nAChRs)) have emerged as targets for the most recently developed compounds (Bloomquist 1996; Zlotkin 1999; Narahashi 2000).
Our interests lie in studying the link between brain nAChRs and the development of Alzheimer’s, where biochemical analysis of brains of patients with AD reveal deficits in nAChR levels (Buckingham, SD. et al. Pharmacological Reviews, 2009; 61 (1) 39-61) and also the development of specific novel insecticides to more successfully treat diseases spread by parasitic nematodes, ticks or other threats to human, animal and crop health (Partridge, FA et al, PLoS NTD; 2017 doi:10.1371/journal.pntd.0005359; Furutani, S. et al, IJP, 2018; 8 (2) : 350-360; Matsuda, K. et al, Ann. Rev. Pharm. and Tox., 2020; 60:241-255).
To assist in our understanding of neuroactive compounds, we approach the study of ligand-gated ion channels using advanced electrophysiology experiments, using voltage- and patch-clamp techniques. However, we are also now pursuing molecular cloning and functional expression of wild-type and mutant ion channel subunits to further our knowledge.
 Close
Close