|
Contact Info :
|
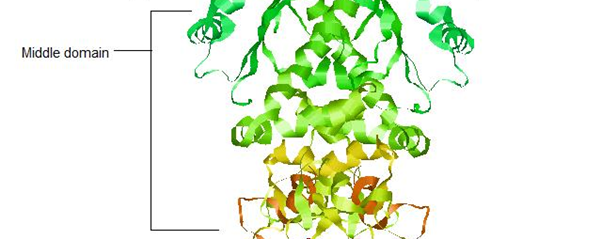
HSP90 - Middle Domain
The middle domain on Hsp90 is implicated in binding to the client protein – for example, PKB. Shown below is the amphipathic loop (residue 329-339) implicated in binding to the client protein.
The loop is present on both protomers of the dimer, but due to high disorder only one is shown in the crystal structure.
|