Coronary Artery Diseases
Coronary artery diseases are one of the largest killers in the UK and worldwide. They are for example associated with approximately 64,000 deaths in the UK in 2020 according to the statistics published by the BHF. Whilst the advancement of in vivo measurement methods such as CT, MR and intravascular images enabled better diagnostics of the disease, computational modelling is expected to complement those measurements by providing biomarkers that are not directly available from in vivo measurement. Ultimately, an integrated multi-modality systems - including computational modelling - could allow prediction of disease progression thus stratifying patients at risk, and personalising the treatment. Biomechanical analysis of coronary artery diseases is the main research target of our research group and we work on this topic in strong partnership with UK and international hospitals. We also work with national and international consortia of clinicians and engineers (Gijsen et al. Eur Heart J 40(41), 2019), to understand pathophysiological mechanism behind the disease and to develoop next-generation diagnostic platforms.
Prediction of Atherosclerotic Disease Progression and Clinical Events using Haemodynamic Metrics
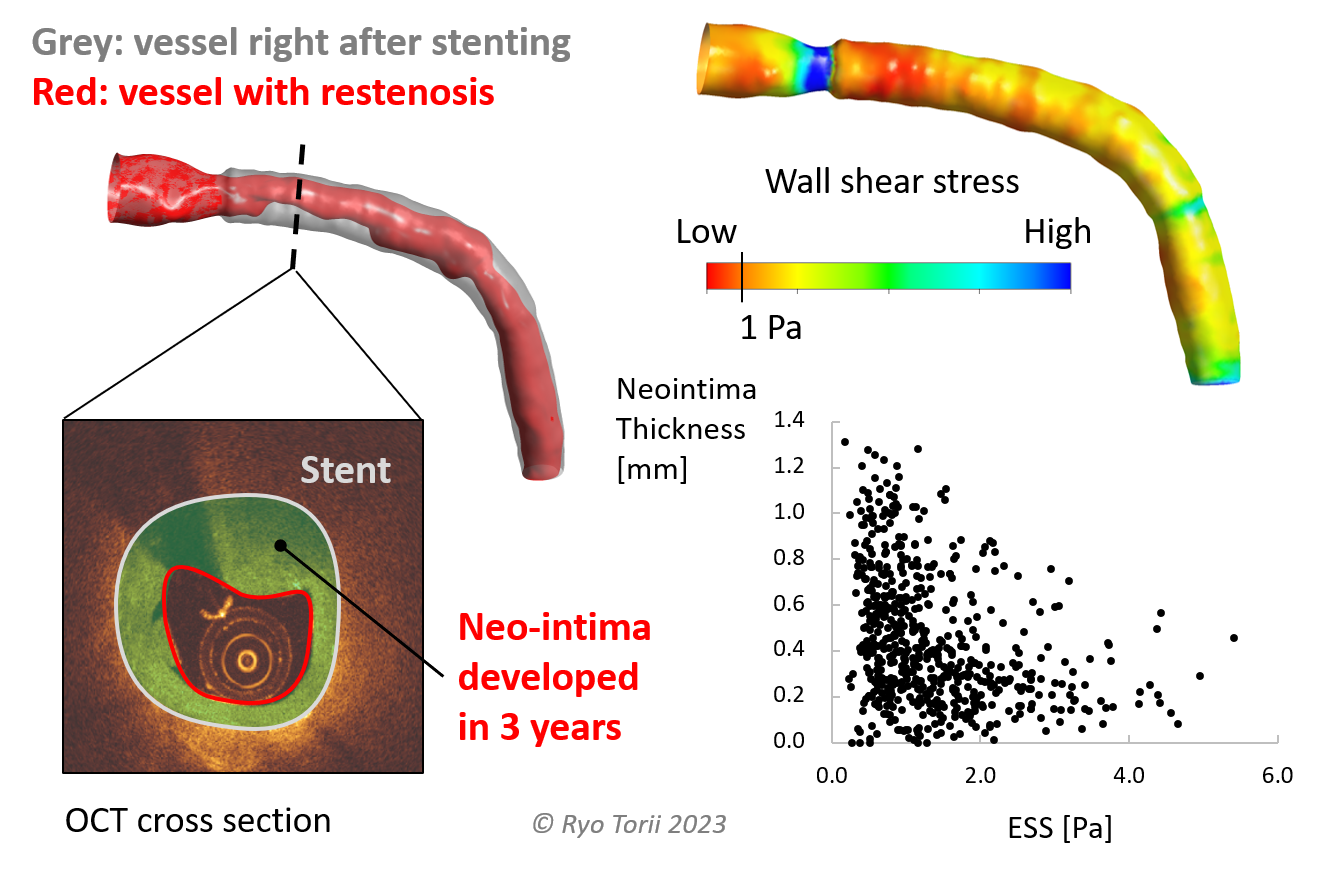 There have been many studies conducted on prediction of coronary atherosclerotic diseases, both development and clinical event such as myocardial infarction. PREDICTION study in 2012 (Stone et al. Ciruclation 126(2), 2012) is an excellent example in which blood flow and resultant wall shear stress (WSS) on the endothelial cell layer were analyised for 506 patients using computational fluid dynamics (CFD) as a "measurement tool". The results demonstrated the value of CFD-derived WSS in predicting progression of atherosclerotic plaques, in addition to plaque burden directly measured from intravascular images. Since then, many studies have been carried out in similar directions, investigating potential association between haemodynamic/biomechanical metrics and clinical events, icluding ours [1]. In such studies, an important consideration is to set the right level of model complexity such that the analyses can capture the key physiological characteristics but be conducted within the timeframe that is short enough and allows investigation of hundreds of patients. To address this, we developed a graphical interface program to streamline the analysis with minimal human input. This platform facilitated a number of studies involving >100 patients/lesions [1-3], also benefiting from simplified modelling analysis conditions (angiogram-based anatomical model and patient-specific but steady flow conditions [2]). Our studies so far demonstrated that such analyses is capable of predicting plaque progression both in native [3] and stented vessels [4], and clinical events in native vessels [1,2]. We are working further on this to make the model more representative, increase predictive capability and accelerate analyses towards analyses in more clinically-viable timeframe.
There have been many studies conducted on prediction of coronary atherosclerotic diseases, both development and clinical event such as myocardial infarction. PREDICTION study in 2012 (Stone et al. Ciruclation 126(2), 2012) is an excellent example in which blood flow and resultant wall shear stress (WSS) on the endothelial cell layer were analyised for 506 patients using computational fluid dynamics (CFD) as a "measurement tool". The results demonstrated the value of CFD-derived WSS in predicting progression of atherosclerotic plaques, in addition to plaque burden directly measured from intravascular images. Since then, many studies have been carried out in similar directions, investigating potential association between haemodynamic/biomechanical metrics and clinical events, icluding ours [1]. In such studies, an important consideration is to set the right level of model complexity such that the analyses can capture the key physiological characteristics but be conducted within the timeframe that is short enough and allows investigation of hundreds of patients. To address this, we developed a graphical interface program to streamline the analysis with minimal human input. This platform facilitated a number of studies involving >100 patients/lesions [1-3], also benefiting from simplified modelling analysis conditions (angiogram-based anatomical model and patient-specific but steady flow conditions [2]). Our studies so far demonstrated that such analyses is capable of predicting plaque progression both in native [3] and stented vessels [4], and clinical events in native vessels [1,2]. We are working further on this to make the model more representative, increase predictive capability and accelerate analyses towards analyses in more clinically-viable timeframe.
Members involved: Dr Ernest Lo, Salwa Alamir, Dr Enhui Yong
Main collaborators: Barts Heart Centre, NUI Galway (CORRIB Core Lab), University Hospital of Bern
Funding support: EPSRC, NIHR BRC, BHF
- Relevant key publications:
- Tufaro et al. Atherosclerosis 322, 2021.
- Bourantas et al. JACC: Cardiovascular Imaging 13(10), 2020.
- Bourantas et al. Eur Heart J Cardiovascular Imaging 20(3), 2019.
- Torii et al. Intl J Cardiology 272,2018.
Computational Methods behind CT-based FFR Calculation
 Derivation of fractional flow reserve (FFR) using CFD and CT-based vascular anatomical model has been highly successful in assessing the haemodynamic severity of coronary atherosclerotic narrowing. The pioneering HeartFlow FFRCT (Taylor et al. JACC 61(22), 2013.) is now recommended in NICE guideline, and there are many others following the success. At the same time, because this approach is based on computational modelling, model assumptions are inevitable and many efforts have been dedicated to refine the computational framework towards higher level of physiological representation. We have been focusing on this aspect and investigating the impacts of inflow and outflow boundary conditions [5,6]. One of the common assumptions is the hyperaemic response of the myocardium, which assumes the resistance of vascular bed is reduced to 30% of its normal state. To investigate the impact of this, we reflected patient-specific myocardial perfusion and hyperaemic response in our model by integrating the information available from PET images of the same patient. The results showed that the variability of the FFR arising from the variability of myocardial perfusion could be significant, especially for those who has a haemodynamically severe narrowing (i.e. low FFR). Also, for those who have borderline FFR values, the FFR could vary across the diagnostic threshold of 0.8 [5]. It is important to note that CT-based FFR assessment is often used as a screening tool rather than final treatment decision-making tool, therefore such a variability does not necessarily raise an immediate concern of misdiagnosis. At the same time, more refined models would increase the applicability of such approach and eventually reduce patient burden, due to the fact that CT-based FFR assessment being non-invasive, which is the main drive for us to continue our effort in this arena.
Derivation of fractional flow reserve (FFR) using CFD and CT-based vascular anatomical model has been highly successful in assessing the haemodynamic severity of coronary atherosclerotic narrowing. The pioneering HeartFlow FFRCT (Taylor et al. JACC 61(22), 2013.) is now recommended in NICE guideline, and there are many others following the success. At the same time, because this approach is based on computational modelling, model assumptions are inevitable and many efforts have been dedicated to refine the computational framework towards higher level of physiological representation. We have been focusing on this aspect and investigating the impacts of inflow and outflow boundary conditions [5,6]. One of the common assumptions is the hyperaemic response of the myocardium, which assumes the resistance of vascular bed is reduced to 30% of its normal state. To investigate the impact of this, we reflected patient-specific myocardial perfusion and hyperaemic response in our model by integrating the information available from PET images of the same patient. The results showed that the variability of the FFR arising from the variability of myocardial perfusion could be significant, especially for those who has a haemodynamically severe narrowing (i.e. low FFR). Also, for those who have borderline FFR values, the FFR could vary across the diagnostic threshold of 0.8 [5]. It is important to note that CT-based FFR assessment is often used as a screening tool rather than final treatment decision-making tool, therefore such a variability does not necessarily raise an immediate concern of misdiagnosis. At the same time, more refined models would increase the applicability of such approach and eventually reduce patient burden, due to the fact that CT-based FFR assessment being non-invasive, which is the main drive for us to continue our effort in this arena.
Members involved: Dr Ernest Lo, Dr Enhui Yong
Main collaborators: UCL Hospital (Nuclear Medicine), Barts Heart Centre, UCL Cardiovascular Science, Royal Free Hospital
Funding support: EPSRC (i4health CDT), BHF
- Relevant key publications:
- Lo, Menezes and Torii. Fluids 4(2), 2019.
- Lo, Menezes and Torii. Med Eng & Phys 76, 2020.
Fluid and Solid Mechanical Analysis of Stented Coronary Vessels
 Most of the patients suffer from severe coronary artery atherosclerotic narrowing undergo percutaneous coronary intervention (PCI) with stent(s) (Bhatnagar et al. Heart 102(24), 2016). Although stent implantation has a long history and is a successful and standard clinical treatment, chances of stent failure is not zero: stent thrombosis and in-stent restenosis - two major modes of stent failure - are reported to occur approximtely 1% (or less) and 5% respectively post-PCI (Byrne et al. Eur Heart J 36(47), 2015). Considering the total number of PCI per year being large, 1%/5% risk affects a large number of patients. We investigated in-stent restenosis in association with biomechanical environment such as WSS, as part of our effort to predict atherosclerotic disease progression [4], also with bioresorbable scaffolds [7]. Additionally, since stents are mostly apposed and/or embedded in the vessel wall, we study their solid mechanical interaction with the vessel tissue by using finite element analyses [8]. Combined analysis of biomechanical metrics could potentially highlight patients at high risk of restenosis and plaque rupture in the future.
Most of the patients suffer from severe coronary artery atherosclerotic narrowing undergo percutaneous coronary intervention (PCI) with stent(s) (Bhatnagar et al. Heart 102(24), 2016). Although stent implantation has a long history and is a successful and standard clinical treatment, chances of stent failure is not zero: stent thrombosis and in-stent restenosis - two major modes of stent failure - are reported to occur approximtely 1% (or less) and 5% respectively post-PCI (Byrne et al. Eur Heart J 36(47), 2015). Considering the total number of PCI per year being large, 1%/5% risk affects a large number of patients. We investigated in-stent restenosis in association with biomechanical environment such as WSS, as part of our effort to predict atherosclerotic disease progression [4], also with bioresorbable scaffolds [7]. Additionally, since stents are mostly apposed and/or embedded in the vessel wall, we study their solid mechanical interaction with the vessel tissue by using finite element analyses [8]. Combined analysis of biomechanical metrics could potentially highlight patients at high risk of restenosis and plaque rupture in the future.
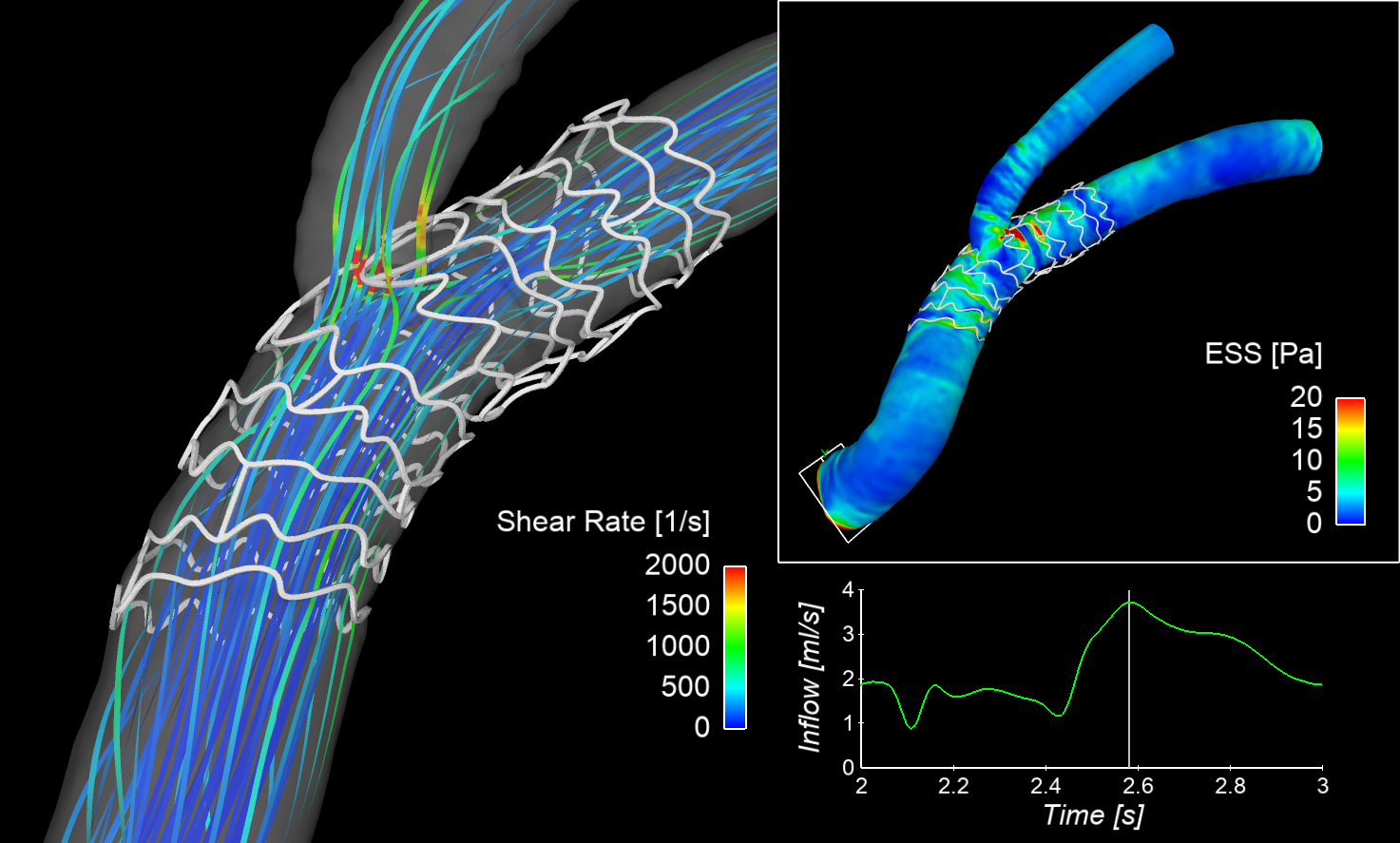
For stent thrombosis, malapposed struts are considered as an important factor since they create a region of high shear rate in the flow that could activate platelet and promote blood clots. We study strut malapposition and potential thrombogenic haemodynamic environment using simple 2D flow analysis [9], benchtop experiments [10] and also based on in vivo images in combination with 3D CFD [11]. CFD can clearly depict regions of high shear rate in blood stream, and our early finding suggests such a region is associated with actual location of thrombosis in vivo [11]. CFD is therefore shown to be a powerful tool in evaluating post-stenting biomechanical environment, could be used to assess haemodynamic performance of different stants/scaffolds [12]. Use of computational modelling to virtually evaluate the efficacy of medical devices - in its development - is indeed pursued as a virtual (in silico) clinical trial tool (Pappalardo et al. IEEE J Biomed Health Info 26(11), 2022) and also by medical regulatory authorities (e.g. FDA).
Main collaborators: Barts Heart Centre, NUI Galway (CORRIB Core Lab), National Heart Centre Singapore
Funding support: MRC
